Relationship between patient-generated subjective global assessment and survival in patients in palliative care
Introduction
In 2012, there were 14.1 million new cancer cases and 8.2 million cancer deaths worldwide (1). According to estimates, there will be approximately 600,000 new cancer cases in Brazil in 2016 and 2017. In addition to non-melanoma skin cancer, which will be responsible for 180,000 new cases, there will be approximately 420,000 new cancer cases (2). According to estimates, in 2030, the global occurrence of new cancer cases and cancer deaths will be 21.4 million and 13.2 million, respectively, which is a result of population ageing and a reduction of child mortality and infectious diseases in developing countries (1).
The majority of cancers are diagnosed when the disease is at a locally advanced and/or disseminated stage, given that initial tumours usually do not produce symptoms that justify a check-up. Therefore, early detection measures are of particular importance because treatment potentially leads to cures only at the initial clinical stages (3). When a chance of recovery is exhausted, the palliative care is employed to enhance treatment (4). Specifically, palliative care is characterised by a set of measures aimed at controlling pain and other symptoms, preventing and relieving suffering, and handling spiritual and psychosocial matters. Hence, when a prognosis is limited, treatment focuses on the quality of life of terminal cancer patients and their family (5).
By providing a comprehensive approach, respecting the desires and needs of the patients and their family, controlling symptoms, enabling socialisation, and resolving pending matters, palliative care helps mitigate the detrimental effects of disease progression and thus improves quality of survival (4). In this context, the Karnofsky Performance Status (KPS) is an important tool to assess and follow-up the functional capacity of terminal cancer patients. The KPS was initially developed in 1940 to assess the effects of chemotherapy on the functional capacity of cancer patients (6). Symptoms such as alterations in taste, nausea, vomiting, early satiety, diarrhoea, constipation, anorexia and weight loss, attributed to collateral damage of treatment or to disease progression itself, affect patient functional capacity and have a negative impact on quality of life. Thus, appropriate symptom management is important in palliative care (7).
Functional capacity and survival are intimately related to the nutritional status of terminal cancer patients (8). Cancer cachexia is characterised by systemic inflammation, negative protein balance and involuntary loss of lean body mass, with or without wasting of adipose tissue. Furthermore, cancer cachexia is present in the majority of terminal cancer patients and is responsible for approximately 22% of deaths in this patient group (9,10).
Aiming to evaluate nutritional status, Detsky et al. (11) have developed the Subjective Global Assessment (SGA), a standardised questionnaire validated as a method to identify surgical patients at nutritional risk or with established malnutrition. However, its use has been adapted to several clinical conditions. This instrument exhibits a good association with morbidity and with anthropometric and laboratory parameters (11). Based on the SGA, Ottery (12) created the Patient-Generated Subjective Global Assessment (PG-SGA), which has been translated and validated for the Portuguese language (13). This is an adaptation of the SGA specific for cancer patients. In addition to group classification resulting from the SGA, the PG-SGA has numeric scores (10). A score is attributed to each component of the PG-SGA according to the impact of symptoms on the nutritional status (14). The sum of the scores then provides guidance with respect to the required level of intervention (14). The PG-SGA has a high degree of sensitivity (98%) and specificity (82%) compared to other tools for the evaluation of the nutritional status of patients with different cancer types and stages (15). Because it relates to the reduction of quality of life, this tool might be useful not only for curative treatment but also during palliative care.
In addition to evaluating the nutritional status of cancer patients, the PG-SGA, combined with the KPS score, potentially exhibits a prognostic value and allows for specific interventions according to disease progression during palliative care. Thus, the objective of the present study was to describe the nutritional profile of terminal cancer patients and to evaluate the relationship among PG-SGA score, clinical-functional characteristics and survival.
Methods
Patients
The present work is a retrospective cohort study with data collected between May 2009 and May 2015 at the Centre for Palliative Care of the Pedro Ernesto University Hospital, State University of Rio de Janeiro. All patients ≥18 years of age who had their PG-SGA score assessed in the aforementioned period were included. Patients with incomplete assessments, with no classification or with incomplete questionnaires and patients or accompanying persons who had trouble communicating were excluded. The protocol was approved by the Research Ethics Committee of the Pedro Ernesto University Hospital, in accordance with the provisions of the Declaration of Helsinki. All patients signed an informed consent form.
Body mass index
Nutritional status was assessed by calculating the body mass index (BMI) during the first medical consultation, when patients still exhibited clinical and functional conditions for measurements. Participants had their body weight and height measured with an anthropometric scale (MIC 2/A 200 kg × 100 g Micheletti mechanical platform scale, São Paulo, Brazil). Based on the obtained data, the BMI was calculated, and patients were classified into three groups: underweight (<18.5 kg/m2), eutrophic (18.5 to 28 kg/m2), and overweight (>28 kg/m2).
Patient-generated subjective global assessment
A nutritionist who had experience and was trained to use the tool administered the PG-SGA on patients during the first medical consultation. The first section of the tool was composed of questions to be completed by patients or their family and was related to body weight, food intake, daily symptoms of the gastrointestinal tract that persisted for more than two weeks, and functional capacity. In the second section, data on the disease and its relation to the nutritional needs, metabolic needs and physical exam were obtained, and patients were classified into groups (SGA-A: well nourished; SGA-B: suspected malnutrition or moderately malnourished; and SGA-C: severely malnourished) and score ranges (score 0–1: no need for nutrition intervention; score 2–3: patient and family education with pharmacological interventions; score 4–8: nutrition intervention; and score ≥9: critical need for symptom control and/or options for nutrition intervention) (13).
Karnofsky Performance Status (KPS)
The KPS score was obtained by professionals of the multidisciplinary team by applying questions and direct observations regarding functionality. This is a scoring system that classifies patients on a scale from zero to 100. Specifically, a score of 100 represents a good state of health, and zero represents death (6). The KPS score is categorised into three ranges: <40 (patient unable to care for self, requiring equivalent of institutional or hospital care; the disease may be progressing rapidly), 40–70 (unable to work, able to live at home, and able to care for most personal needs; a varying degree of assistance is needed), and >70 (able to carry on normal activity and to work; no special care is needed).
Statistical analysis
The sample size was calculated using MedCalc version 8.2 (MedCalc Software, Mariakerke, Belgium). A minimum of 80 cases were required to test the alternative hypothesis that the correlation coefficient (correlation between the PG-SGA and KPS scores) was higher than 0.40 (or less than –0.40), assuming a type I error of 5% and a type II error of 20%.
The time of cancer mortality was determined considering the interval between the admission examination and the informed date of death, or the last telephone contact with the patient or family if there was no death report in the record, or the date of the last examination when there was no information on the date of death and it was not possible to get in touch with the patient or his/her family. The Kolmogorov-Smirnov test was performed, and the continuous variables age, KPS, and PG-SGA exhibited a normal distribution, whereas time of survival exhibited a non-normal distribution. In the sample description, data are expressed as the mean ± standard deviation, or median (minimum and maximum values) for numeric variables, according to the normality of variables, and proportional to categoric variables. Multiple comparisons of numeric variables between three or more groups were performed via analysis of variance (ANOVA). Parametric correlations were tested by means of Pearson’s correlation analysis, and non-parametric correlations were tested with Spearman’s correlation analysis. The Kaplan-Meier estimate was used to calculate the time of survival, and the Log-Rank significance test evaluated the equality of distribution of survival. The probability of survival was evaluated with the variables PG-SGA score (categorised into three ranges: 0–3; 4–8; and ≥9) and KPS score (categorised into three percent ranges: <40; 40–70; and >70). Data analysis was performed using SPSS software, version 17 (SPSS, Chicago, IL, USA). A level of significance of 5% was adopted for all statistical tests.
Results
Social and clinical characteristics
The studied sample was composed of 104 patients with a mean age of 64.9±13 years, of which 59.6% were men. Regarding education level, only 5.8% had a bachelor’s degree or higher. With respect to religion, most reported to be Catholic (65.4%), and 10.6% had no religious affiliation. Most of the patients (56.7%) reported one or more comorbidities, of which the most common were hypertension and diabetes. Regarding the primary tumour site, the most prevalent sites were the genitourinary system (19.2%), lungs (18.3%) and head and neck (18.3%). Distant metastases were reported by 70.3%, with the bones, liver and lungs most commonly affected. Social and clinical patient characteristics and the site of origin of primary lesions and that of metastases are listed on Table 1.

Full table
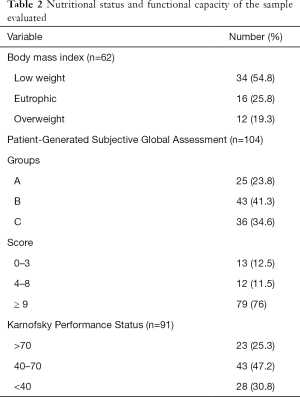
According to their PG-SGA, 24%, 41.3% and 34.6% of the patients were classified as SGA-A, SGA-B and SGA-C, respectively. Regarding the numeric PG-SGA, 76% exhibited a score ≥9. With respect to a history of weight loss evaluated in the PG-SGA, 19.2% of the patients were not able to recall their weight one and six months ago, and, among those who were able to provide this information, 43.3% exhibited weight loss in one or both time periods. The mean PG-SGA score was 14±7.4. The mean KPS score was 58.6±17.3, among which a KPS score ≤40 was found in 30.8% of the patients (Table 2). There was a statistically significant inverse correlation between the PG-SGA and KPS scores (r=–0.432, P<0.001). In the analysis of the three PG-SGA groups, we found that the worse the classification of the nutritional status was, the lower the KPS score (P<0.001).
Full table
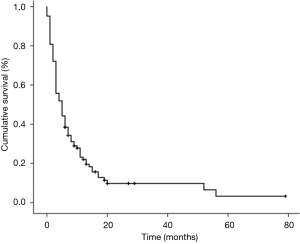
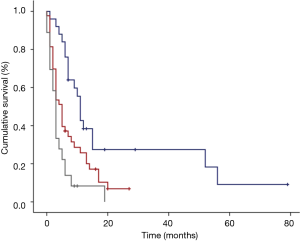
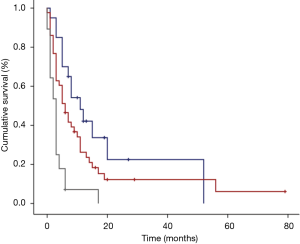
The median time of cancer mortality in the studied sample was 5 (3.3–6.7) months (Figure 1). There was a statistically significant difference in the time of survival between the PG-SGA classifications. Figure 2 displays the survival curves according to the three PG-SGA groups. According to this classification, the median survival of the SGA-A, SGA-B and SGA-C groups was 11 (9.5–12.5), 5 (3.6–6.5) and 3 (2.4–3.6) months, respectively (P<0.001). There was a statistically significant difference in the time of survival between the PG-SGA score ranges. Figure 3 shows the survival curves according to the PG-SGA score ranges; according to this classification, the median survival of patients within score ranges of 0–3, 4–8 and ≥9 was 7 (1–13), 11 (3.1–19) and 3 (1.9–4.1) months, respectively (P=0.036). There was a statistically significant difference in the time of survival between the classifications of the KPS score. Figure 4 displays the survival curves according to the three KPS score groups. According to this classification, the median survival of the <40, 40–70 and >70 groups was 3 (2.2–3.8), 6 (3.6–8.4) and 11 (6.1–16) months, respectively (P<0.001).

In the analysis concerning the site of the disease and the BMI, there was a difference in the meantime of survival, albeit with no statistical significance. The mean time of survival was lower for patients with lung tumours and for those classified as underweight (Table 3).
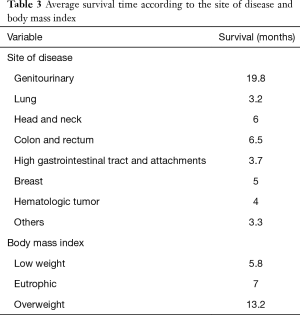
Full table
Discussion
Prognostic evaluation is an important task for health professionals involved in terminal cancer patient care. It is necessary for decision making regarding treatment and to improve quality of life (16).
In the studied sample, male patients were predominant, and the mean age was >60 years, which is consistent with other studies on terminal cancer patients (17-19). With respect to education level, the majority had an incomplete primary school education. Notably, education level is an indicator of poverty and is related to a lack of knowledge, difficulty in accessing the health care system, late diagnosis and advanced disease stages (20). When asked about the religion they practised, most patients declared to be Catholic, followed by evangelic and no religious affiliation. The focus on spiritual and religious patient matters must occur at the beginning of assistance, considering and respecting patient beliefs, to take the necessary measures for the resolution of possible intercurrences of the patient, family and team (4).
With respect to the primary site of the disease, the head and neck was among the most prevalent, with a mean time of survival of six months, which is consistent with the previously reported median survival of four to seven months among patients with epidermoid head and neck cancer who developed metastatic disease (21). Interestingly, the sites of metastases may have an impact on survival, and liver metastases are significantly prognostic (22). Similar to our findings, Chuang et al. (17) and Manfro et al. (19) have shown that the most predominant metastases among patients under palliative care are located in the bones, lungs and liver and are responsible for significant functional limitations, such as pain, dyspnoea, difficulty in locomotion and ascites.
Cancer treatment and disease progression produce symptoms that can reduce food intake and, thus, alter the nutritional status and negatively affect the quality of life of the terminal cancer patient (23). In the present study, the evaluation of the BMI revealed that more than half of the patients who had their index calculated in the first medical consultation were underweight. Of note, the BMI was not calculated in as many as 40.4% of the patients due to their difficulties in walking and standing on the scale. The PG-SGA, which was evaluated in the entire sample, exhibited a nutritional risk or the onset of moderate/severe malnutrition in 75.9% of the patients and is thus an important tool for this population, given that it is a subjective method that considers broader questions, such as the presence of symptoms with nutritional impact, functional capacity, physical exam, and alterations in body weight (13). Weight loss occurs due to a negative energy balance, which is a result of reduced food intake and metabolic changes caused by the disease (24). Interestingly, approximately 80–90% of terminal cancer patients exhibit significant weight loss, of which approximately 25% die due to cancer anorexia and cachexia syndrome (25).
Malnutrition has a negative impact on both patient well-being and the clinical evolution of the disease and is, thus, a factor of poor prognosis that significantly affects survival (25). In the present study, the reduced median time of cancer mortality might be due to the advanced stage of the disease present in the majority of the patients and to the several symptoms related to disease progression that negatively impact quality of life, functional capacity, and nutritional status and reduce survival. Tan et al. (26) and Gupta et al. (18) have found that terminal cancer patients with SGA-C had a lower median survival than those with SGA-B or SGA-A, thus reinforcing the importance of implementing the PG-SGA in cancer treatment centres, given that it provides useful information on prognosis. In the present study, both SGA-C patients and those with a score ≥9 in the numeric PG-SGA exhibited a median survival of three months, supporting the fact that symptoms caused by the evolution of the disease are directly associated with reduced functional capacity and prognosis. However, patients within the score range of 4–8 exhibited higher survival compared with those within the score range of 0–3, which might be explained, at least in part, by the positive effect of the interventions performed during palliative treatment. The PG-SGA score also demonstrated the critical need for immediate intervention, including nutritional intervention and symptom control in most patients. This result is consistent with the study by Shahmoradi et al. (27), which found that almost all patients needed some type of recommendation to improve their nutritional status and quality of life and that the higher the PG-SGA score was, the worse the individual outcome. However, it is important to note that the nutritional approach in palliative care must envision overall well-being rather than reversing the nutritional status.
In addition to the nutritional status, the functional capacity of terminal cancer patients is considered an important prognosis factor, given that one of the main concerns in the treatment of these patients is to maximally maintain their autonomy in everyday activities (28). In this context, a low KPS score has been recognised as a predictor of worse survival, especially among patients with a score ≤40 (29). Several studies have shown that the KPS score is a tool with high precision for medical estimates regarding life expectancy (30-32). In the present study, the PG-SGA and KPS exhibited a statistically significant inverse correlation, suggesting that both can be used in the evaluation of patients under palliative care, given that the obtained information is complementary and provides guidance for the adequate measures to assess for each patient. We think that the KPS should be applied in all medical consultations, to compare the functional status of patients at the intervals between exams and, thus, facilitate the evaluation of the measures chosen for each patient by the multiprofessional team.
Based on the evaluation performed with the PG-SGA and KPS, patients must receive nutritional guidance and strategies on increasing nutrient fractionation, calorie density of meals, nutritional supplement intake and best-tolerated food consistency. Furthermore, patients should then be offered their preferred food or meals that may help improve symptoms, provided they are able to receive them and conditions are re-evaluated at each follow-up. In a study by Baldwin et al. (33), oral nutritional supplementation in malnourished terminal cancer patients was associated with a significant increase in energy intake, beneficial effects on appetite and improved global quality of life. Despite the great usefulness of these tools, each measure must always be individualised, considering the peculiarities of each patient, including those scoring ≥9 on the PG-SGA, in which case the multiprofessional team is advised to reconsider the recommendations exposed by the tool and to make adaptations whenever needed. Continuous monitoring of cancer survival must become an inexhaustible source of information for health professionals and a stimulus to improve the political and health care systems (34). Further research in this field is imperative to provide insight and broaden knowledge on the matter to ensure a more reliable intervention that aims to contribute to an improved quality of life and, thus, to enhance the survival of terminal cancer patients under palliative care.
Conclusions
The nutritional status of terminal cancer patients under palliative care is thought to have a great influence on quality of life and survival because it is responsible for various functional limitations exhibited by these individuals. Thus, continuous monitoring, including nutritional and functional evaluations, is necessary. The present study found that at the first medical consultation, most of the patients exhibited nutritional risk or moderate/severe malnutrition in need of immediate intervention for symptom control and of specific nutritional guidance, according to an analysis of the PG-SGA. Furthermore, the analysis of survival showed that the most severely affected nutritional status, the highest presence of symptoms and most reduced functional capacity were associated with a lower survival time among the studied population. Given that the PG-SGA is a method that identifies and evaluates subjective aspects, such as changes in body weight, the presence of symptoms, food intake, functional capacity and metabolic changes, the assessment allows health professionals to determine the measures to be taken for each patient in a satisfactory manner. Thus, the authors suggest that the PG-SGA should be used not only to evaluate nutritional status but also as a complementary tool to the KPS to evaluate survival in palliative care.
Acknowledgements
This research was supported by Rio de Janeiro State Research Supporting Foundation (FAPERJ).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institution’s Research Ethics Board (No. CAAE- 47829515.0.0000.5259), and written informed consent was obtained from all patients.
References
- Globocan. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Estimativa 2016: Incidência de Câncer no Brasil. 2016. Available online: http://www.inca.gov.br/estimativa/2016/estimativa-2016-v11.pdf
- Castanho IA, Lopes AJ, Koury JC, et al. Relationship between the phase angle and volume of tumours in patients with lung cancer. Ann Nutr Metab 2013;62:68-74. [Crossref] [PubMed]
- Academia Nacional de Cuidados Paliativos. Manual de Cuidados Paliativos ANCP. 2nd ed., August, 2012. Available online: www.paliativo.org.br/dl.php?bid=146
- World Health Organization. National Cancer Control Programmes: Policies and Managerial Guidelines. 2nd ed., Geneva, 2002. Available online: www.who.int/cancer/media/en/408.pdf
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM (ed). Evaluation of Chemotherapeutic Agents in Cancer. Columbia University Press, New York, 1949; 191-205.
- National Cancer Institute. Nutrition in Cancer Care (PDQ®)–Patient Version. 2016. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects/appetite-loss/nutrition-pdq
- Tan BH, Fearon KC. Cachexia: Prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care 2008;11:400-7. [Crossref] [PubMed]
- Skipworth RJ, Stewart GD, Dejong CH, et al. Pathophysiology of cancer cachexia: much more than host-tumour interaction? Clin Nutr 2007;26:667-76. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr 1987;11:8-13. [Crossref] [PubMed]
- Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996;12:S15-9. [Crossref] [PubMed]
- Gonzalez MC, Borges LR, Silveira DH, et al. Validação da versão em Português da Avaliação Subjetiva Global Produzida pelo Paciente. Rev Bras Nutr Clin 2010;25:102-8.
- Ottery FD. Patient-generated subjective global assessment. In: McCallum PD, Polisena CG, editors. The Clinical Guide to Oncology Nutrition. The American Dietetic Association, Chicago, 2000;11-23.
- Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779-85. [Crossref] [PubMed]
- Glare P, Sinclair C, Downing M, et al. Predicting survival in patients with advanced disease. Eur J Cancer 2008;44:1146-56. [Crossref] [PubMed]
- Chuang RB, Hu WY, Chiu TY, et al. Prediction of survival in terminal cancer patients in Taiwan: Constructing a prognostic scale. J Pain Symptom Manage 2004;28:115-22. [Crossref] [PubMed]
- Gupta D, Lammersfeld CA, Vashi PG, et al. Prognostic significance of Subjective Global Assessment (SGA) in advanced colorectal cancer. Eur J Clin Nutr 2005;59:35-40. [Crossref] [PubMed]
- Manfro G, Dias FL, Soareset JRN, et al. Relationship between age, gender, treatment, and disease stage and survival in terminal patients with squamous cell carcinoma of the larynx. Rev Bras Cancerol 2006;52:17-24.
- Kligerman J. Cancer as a health parameter in Brazil. Rev Bras Cancerol 1999;45:5-6.
- Troell RJ, Terris DJ. Detection of metastases from head and neck cancers. Laryngoscope 1995;105:247-50. [Crossref] [PubMed]
- Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer: Pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 2004;22:2395-403. [Crossref] [PubMed]
- Argilés JM, Anker SD, Evans WJ, et al. Consensus on cachexia definitions. J Am Med Dir Assoc 2010;11:229-30. [Crossref] [PubMed]
- Bossola M, Pacelli F, Tortorelli A, et al. Cancer cachexia: it's time for more clinical trials. Ann Surg Oncol 2007;14:276-85. [Crossref] [PubMed]
- Segura A, Pardo J, Jara C, et al. An epidemiological evaluation of the prevalence of malnutrition in Spanish patients with locally advanced or metastatic cancer. Clin Nutr 2005;24:801-14. [Crossref] [PubMed]
- Tan CS, Read JA, Phan VH, et al. The relationship between nutritional status, inflammatory markers and survival in patients with advanced cancer: a prospective cohort study. Support Care Cancer 2015;23:385-91. [Crossref] [PubMed]
- Shahmoradi N, Kandiah M, Peng LS. Impact of nutritional status on the quality of life of advanced cancer patients in hospice home care. Asian Pac J Cancer Prev 2009;10:1003-9. [PubMed]
- Yavuzsen T, Walsh D, Davis MP, et al. Components of the anorexia-cachexia syndrome: gastrointestinal symptoms correlates of cancer anorexia. Support Care Cancer 2009;17:1531-41. [Crossref] [PubMed]
- Cherny N, Fallon M, Kaasa S. Oxford Textbook of Palliative Medicine. 5th ed. Oxford University Press, Oxford, 2015.
- Evans C, McCarthy M. Prognostic uncertainty in terminal care: does the Karnofsky index help? Lancet 1985;1:1204-6. [Crossref] [PubMed]
- Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med 1988;148:1586-91. [Crossref] [PubMed]
- Yates JW, Chalmer B. McKegney. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 1980;45:2220-24. [Crossref] [PubMed]
- Baldwin C, Spiro A, Ahern R, et al. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2012;104:371-85. [Crossref] [PubMed]
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. [Crossref] [PubMed]