Managing chemotherapy-induced nausea and vomiting in head and neck cancer patients receiving cisplatin chemotherapy with concurrent radiation
Introduction
In 2015 the number of new estimated cancer cases in North America was almost 1.9 million, with total deaths estimating roughly seven hundred thousand (1,2). Although head and neck cancer makes up a small proportion of these patients, the burden of the disease is tremendous. The most common treatment for patients with head and neck cancer is platinum chemotherapy with concurrent radiation (3). With the disease having a large impact on quality of life including food consumption, communication, and social interactions, it is critical that the best possible treatment options are available for patients to provide the greatest chance of disease control and optimal quality of life. Determining the most effective anti-emetic combination to prevent chemotherapy induced nausea and vomiting (CINV) is one of the biggest challenges for oncologists and pharmacists. Since the introduction of 5HT3 receptor antagonists (RA), it has been easier to manage the vomiting and nausea experienced by patients during chemotherapy.
While 5HT3-RA are effective at preventing vomiting in the acute setting (<24 hours), they are poor at preventing vomiting and nausea in the delayed phase (24–120 hours) following chemotherapy. For high emetogenic chemotherapy, international guidelines recommend triple drug therapy for prevention of CINV (4,5). For moderate emetogenic chemotherapy (MEC), the most up-to-date guidelines suggest a second-generation 5HT3-RA (palonosetron) with dexamethasone for improved protection (5). Advances in our understanding of the underlying mechanisms of CINV and introduction of new agents have improved our prevention of CINV; however, challenges remain.
Unfortunately in some situations the prescribing of palonosetron may be restricted due to availability and/or reimbursement policies. Patients may be only able to access palonosetron through private drug plans or out-of-pocket payment. When palonosetron is not used the best option is to adhere to the recommendations of the Multinational Association of Supportive Care in Cancer (MASCC) and substitute palonosetron with either ondansetron or granisetron. Although these guidelines are available, clinical adherence is sub-optimal (6). The lack of adherence to antiemetic guidelines could be explained by an institution’s outdated anti-emetic protocol, a physicians’ prescribing preference, reimbursement policies or a patients’ willingness to pay for drug costs. This study was conducted to review the anti-emetic protocols used at our outpatient cancer centre to determine the rate of CINV and number of changes made to anti-emetic prophylaxis on subsequent cycles in head and neck cancer patients receiving platinum therapy with concurrent radiation. Currently CINV in patients being treated for head and neck cancers receiving platinum therapy is only reported in the context of phase II or III clinical treatment trials using general toxicity scales such as the CTCAE (7-11). A further limitation is that these trials typically report worse grades as reported by the patient or as assessed by the oncologist retrospectively.
Methods
Study description
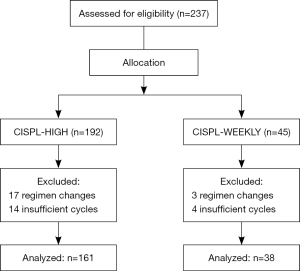
This was a retrospective study of a consecutive cohort of head and neck cancer patients receiving high-dose cisplatin every three weeks or low-dose weekly cisplatin with concurrent radiation treatment initiated between January 2013 and June 2015 in the Odette Cancer Centre. Single high-dose cisplatin (≥50 mg/m2) was considered highly emetogenic, while low-dose weekly cisplatin (<50 mg/m2) was considered moderately emetogenic. Eligible patients were required to have received at least two cycles of the same regimen. Patients were ineligible if they received only one cycle of chemotherapy or had their treatment switched at any point during the study period (Figure 1).
Anti-emetic therapy
Patients’ anti-emetic use was determined by reviewing orders in the centre’s physician order entry system, and instances of nausea and vomiting were determined through review of pharmacy patient profiles and oncologist dictations in the centre’s electronic medical records. Anti-emetic changes were made either after patients discussed their previous cycle with their medical oncologist before each new cycle period or after being followed up with by a pharmacist/pharmacy student 24–48 hours after each cycle was completed. Patients received one of two anti-emetic regimens based on emetogenic risk for cycle 1 (Table 1). These anti-emetic regimens could change in subsequent cycles depending on patient experience and physician’s discretion.

Full table
Study outcomes
The main outcome of interest was complete response rate (no nausea or vomiting) from first day of chemotherapy to 120 hours post-chemotherapy treatment across all cycles of treatment; acute and delayed were not defined separately. We were also interested in the number of changes to patients’ anti-emetic regimens.
Statistical analysis
Descriptive statistics were used to summarize patient demographics. The Fisher exact method was used to compare nausea, CINV (nausea and vomiting), and complete response with age, gender, and emetogenicity. A multivariable logistic regression analysis was also conducted to examine the association between nausea and vomiting with age, gender and emetogenicity of the chemotherapy regimen. A two-tailed P<0.05 was considered as statistically significant. Statistical analysis was performed using SAS software version 9.4 (SAS, Inc., Cary, North Carolina, USA).
Results
Patient characteristics
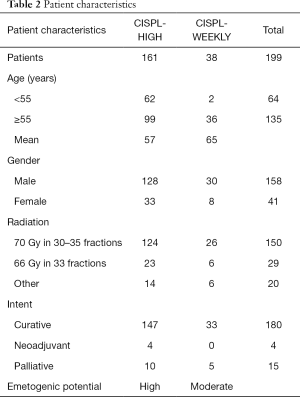
A total of 199 patients, 158 male and 41 female, were divided into two treatment cohorts: CISPL-HIGH (n=161), and CISPL-WEEKLY (n=38). Ninety percent of patients received 66–70 Gy in 30–35 fractions concurrent with chemotherapy (Table 2).
Full table
CISPL-HIGH regimen cohort
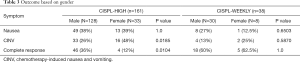
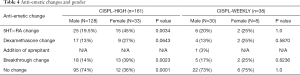
A total of 161 patients, 128 male and 33 female, were included in the analysis. Complete response was achieved in 46 males (36%) and 4 females (12%). CINV occurred in 33 males (26%) and 16 females (48%), while nausea alone occurred in 49 males (38%) and 13 females (39%). Eighty-five percent of patients experienced nausea and/or vomiting in cycle 1 and 14% in cycle 2. Assessment of anti-emetic regimen changes after failure of prophylaxis revealed that 107 patients (66%) had no changes to their regimen. Forty patients had a change in their 5HT3-RA; 27 switches and 13 dose extensions past the two day prescribed period. A change in the duration and/or strength of dexamethasone was seen in 26 patients (16%). All patients received aprepitant for primary prophylaxis of CINV. Breakthrough anti-emetic change occurred in 31 patients (19%) (Tables 3,4).
Full table
Full table
CISPL-WEEKLY regimen cohort
A total of 38 patients, 30 male and 8 female, were included in the analysis. Complete response was achieved in 18 males (60%) and 5 females (62.5%). CINV occurred in 4 males (13%) and 2 females (25%), while nausea alone occurred in 8 males (27%) and 1 female (12.5%). Sixty percent of patients experienced nausea and/or vomiting in cycle 1 and 20% in cycle 2. Assessment of anti-emetic regimen changes after failure of prophylaxis revealed that 28 patients (74%) had no changes to their regimen. Eight patients (21%) had a change in their 5HT3-RA; four switches and four dose extensions past the two day prescribed period. Dexamethasone was added post-chemotherapy to 6 patients (16%) and aprepitant to 1 patient (3%). Breakthrough anti-emetic change occurred in 7 patients (18%) (Tables 3,4).
Males vs. female
Fisher exact test revealed a significant difference between CINV experienced by females (49%) compared to males (26%) receiving high-dose cisplatin (P=0.0185), as well as a significant difference between the complete response rate of males (36%) compared to females (12%) receiving high-dose cisplatin (P=0.01). There were several significant differences with anti-emetic changes for HEC; the number of 5HT3-RA changes for males (19.5%) compared to females (45%) (P=0.0034), as well as the number of breakthrough changes for males (14%) versus females (39%) (P=0.0023). Seventy-four percent of males had no change in their anti-emetic regimen compared to females (36%), which was statistically significant (P=0.0001). Multivariable analysis concluded that males had an odds ratio of 0.39 (95% CI: 0.17–0.89) for nausea or vomiting versus complete response compared to females (P=0.03) (Tables 3-5).

Full table
Age <55 vs. ≥55 years
Fisher exact test revealed no significant differences for the two treatment cohorts between age and either nausea, CINV, or complete response. Multivariable analysis concluded that patients aged 55 or over had an odds ratio of 0.96 (95% CI: 0.49–1.89) for nausea or vomiting versus complete response compared to younger patients (P=0.91) (Tables 5,6).
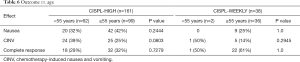
Full table
MEC vs. HEC
Fisher exact test revealed a significant difference between complete response with MEC (61%) and HEC (31%) (P=0.0012). Multivariable analysis concluded that patients receiving HEC had an odds ratio of 3.51 (95% CI: 1.61–7.67) for nausea or vomiting versus complete response compared to MEC (P=0.002) (Tables 5,7).
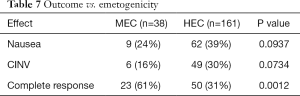
Full table
Breakthrough
Across both treatment groups there were 38 patients with breakthrough anti-emetic changes. Breakthrough medication is defined as additional support after failed first-line prophylaxis resulting in nausea and/or vomiting. Prochlorperazine is the standard breakthrough anti-emetic given with MEC and HEC. When a breakthrough failure occurred olanzapine was substituted for prochlorperazine 62% of the time in the CISPL-HIGH cohort, and 57% in CISPL-WEEKLY cohort.
5HT3-RA change
Out of the 48 patients that had a change to their 5HT3-RA, 17 (35%) had a change in the strength or duration of their ondansetron, three (6%) were switched from ondansetron to palonosetron and 28 patients (58%) were switched from ondansetron to granisetron.
Discussion
Although cisplatin chemotherapy is commonly associated with delayed CINV it is underreported in head and neck cancer patients. Literature searches conducted through PubMed for cisplatin-induced CINV, with or without radiation, reported mainly on breast and lung cancer patients (12-14). We found only one study that reported on anti-emetic efficacy with low-dose cisplatin and concurrent radiation therapy where over 50% of the population was being treated for head and neck cancer (15). Even then, the overall complete response rate (no emesis and no rescue) was compared for the first two cycles only, with aprepitant (cycle 1: 86.4% vs. cycle 2: 83.3%) and without (cycle 1: 72.7% vs. cycle 2: 63.6%). We found one other study by Tsukuda et al. that reviewed anti-emetic efficacy of high-dose cisplatin-based chemotherapy with head and neck cancer patients, but they did not receive concurrent radiation (16). The study used the same definition of complete response as our study, and determined the average overall complete response rate over the 5-day observed period to be 34% (D1: 58.3%, D2: 36.1%, D3: 33.3%, D4/D5: 22.2%). However patients only received granisetron and dexamethasone. In our study the overall complete response rate for MEC and HEC was 61% and 31%, respectively.
A study by Jahn et al. defined complete response as “no emesis and no use of rescue medication”. This is a major challenge for anti-emetic efficacy studies because the use of different complete response definitions makes it difficult to make comparisons across trials. The study reported a complete response rate of 86.4% and 83.3% in cycles 1 and 2, respectively, which is higher than reported in our study. However it is important to note that the study by Jahn et al. used “no use of rescue medication” as a surrogate for no nausea, which is a weak indicator because patients could still be experiencing nausea without wanting to take their breakthrough medications. Patients rate nausea as more problematic than vomiting (17). Therefore, the definition of complete response may need to be modified to incorporate any nausea experienced, not based off breakthrough medication use. Delayed nausea is underestimated by physicians and is poorly observed because it occurs outside of the clinic (18,19). Patient experience is subjective and varies by individual, and thus it is difficult to measure the incidence of nausea. Breakthrough antiemetics are important to manage delayed nausea and prevent vomiting when patients do not have immediate access to their physicians to order more prophylactic antiemetics. In terms of delayed nausea control, there is a lack of clear evidence demonstrating that palonosetron is more effective than first generation 5HT3-RA. This is also the case for aprepitant compared to prochlorperazine (20). We know that dexamethasone added post-chemotherapy provides some control of delayed nausea, but recent studies suggest that olanzapine is the most effective at managing delayed nausea (21,22). In a recent high-dose cisplatin study analysis by Abe et al., olanzapine combined with triple drug therapy resulted in a total control rate (no nausea) of 80.5% and “no significant nausea” rate of 95.5% over the entire treatment phase (0–120 h) (23). A valuable breakthrough trial in 2013 conducted by Navari et al. revealed that olanzapine was three times more effective at treating delayed nausea over metoclopramide for patients that initially failed first-line anti-emetic treatment (24). In our study, olanzapine was the most common switch after failed first-line breakthrough treatment with prochlorperazine.
From the current study we can suggest that our antiemetic protocols are in need of changes. Triple drug therapy for high emetogenic chemotherapy is absolutely necessary to manage CINV in the acute and delayed phase. Additional support in the delayed phase is recommended with the optimal choice being olanzapine for breakthrough nausea. Although ondansetron and granisetron are similar in controlling CINV (25,26) the present study suggests a change to granisetron as the standard of care may be acceptable. If ondansetron is used then a cross-over to granisetron after failure may be used (27).
This study was limited by its retrospective design. The number of patients who experienced acute or delayed nausea and vomiting could not be accurately determined as it is not recorded consistently in patient profiles and dictations. Thus, only the occurrence of nausea and vomiting could be obtained. Follow-up with patients in real-time after making changes to their anti-emetic regimen was not feasible and so it could not be accurately confirmed if patients took their breakthrough medication. Therefore, it was unknown whether the double/triplet-therapy or the breakthrough anti-emetics controlled CINV. Additionally, we were unable to determine the severity of nausea or vomiting using an assessment tool due to the study’s retrospective design. Although there were limitations, the outcomes were in line with potential risk factors for CINV.
Overall this study provided a Canadian perspective into the trials and tribulations of anti-emetic management with chemotherapy-induced nausea and vomiting (CINV). Since palonosetron is not commonly prescribed at our cancer centre it was necessary to review the anti-emetic protocols to determine the best possible treatment regimen. Moving forward, the first step is to follow MASCC guidelines more closely for scheduling 5HT3-RA and dexamethasone to improve complete response rates. A change to olanzapine as the primary breakthrough anti-emetic may help improve management post-chemotherapy when nausea or vomiting occurs. Future studies will need to be conducted once changes are made to our protocols to determine efficacy and safety, and make a stronger recommendation. From our view this is the first and largest study outside of a clinical trial setting where the primary objective focuses on nausea and vomiting, not progression free survival targeting the head and neck population.
Acknowledgements
Dr. Carlo DeAngelis is the senior author and Principal Investigator of this study. We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee of Sunnybrook Health Sciences Centre (No. 218-2014).
References
- American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society, 2015.
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society, 2015.
- Visacri MB, Ferrari GB, Dias P, et al. Quality of Life of Patients with Squamous Cell Carcinoma of the Head and Neck Receiving High-Dose Cisplatin Chemotherapy and Radiotherapy. South Med J 2015;108:343-9. [PubMed]
- MASCC/ESMO Anti-emetic guideline. V.1.2. (2016). Available online: http://www.mascc.org/assets/Guidelines-Tools/mascc_antiemetic_guidelines_english_2016_v.1.2.pdf
- Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol 2003;21:4112-9. [Crossref] [PubMed]
- França MS, Usón PL Junior, Antunes YP, et al. Assessment of adherence to the guidelines for the management of nausea and vomiting induced by chemotherapy. Einstein (Sao Paulo) 2015;13:221-5. [Crossref] [PubMed]
- Ohno T, Wakatsuki M, Thinh DH, et al. Concurrent chemoradiotherapy for T3-4 and N0-1 nasopharyngeal cancer: Asian multicenter trial of the Forum for Nuclear Cooperation in Asia. J Radiat Res 2016;57:44-9. [Crossref] [PubMed]
- Homma A, Inamura N, Oridate N, et al. Concomitant weekly cisplatin and radiotherapy for head and neck cancer. Jpn J Clin Oncol 2011;41:980-6. [Crossref] [PubMed]
- Gupta T, Agarwal JP, Ghosh-Laskar S, et al. Radical radiotherapy with concurrent weekly cisplatin in loco-regionally advanced squamous cell carcinoma of the head and neck: a single-institution experience. Head Neck Oncol 2009;1:17. [Crossref] [PubMed]
- Hitt R, Grau JJ, López-Pousa A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 2014;25:216-25. [Crossref] [PubMed]
- Ang KK, Harris J, Garden AS, et al. Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: radiation therapy oncology group phase II trial 99-14. J Clin Oncol 2005;23:3008-15. [Crossref] [PubMed]
- Schwartzberg L, Barbour SY, Morrow GR, et al. Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting (CINV). Support Care Cancer 2014;22:469-77. [Crossref] [PubMed]
- Kitazaki T, Fukuda Y, Fukahori S, et al. Usefulness of antiemetic therapy with aprepitant, palonosetron, and dexamethasone for lung cancer patients on cisplatin-based or carboplatin-based chemotherapy. Support Care Cancer 2015;23:185-90. [Crossref] [PubMed]
- Tanioka M, Kitao A, Matsumoto K, et al. A randomised, placebo-controlled, double-blind study of aprepitant in nondrinking women younger than 70 years receiving moderately emetogenic chemotherapy. Br J Cancer 2013;109:859-65. [Crossref] [PubMed]
- Jahn F, Riesner A, Jahn P, et al. Addition of the Neurokinin-1-Receptor Antagonist (RA) Aprepitant to a 5-Hydroxytryptamine-RA and Dexamethasone in the Prophylaxis of Nausea and Vomiting Due to Radiation Therapy With Concomitant Cisplatin. Int J Radiat Oncol Biol Phys 2015;92:1101-7. [Crossref] [PubMed]
- Tsukuda M, Ishitoya J, Mikami Y, et al. Antiemetic effects of granisetron and dexamethasone combination therapy during cisplatin-containing chemotherapy for head and neck cancer: dexamethasone dosage verification trial. Int J Clin Oncol 2009;14:337-43. [Crossref] [PubMed]
- Hernandez Torres C, Mazzarello S, Ng T, et al. Defining optimal control of chemotherapy-induced nausea and vomiting-based on patients’ experience. Support Care Cancer 2015;23:3341-59. [Crossref] [PubMed]
- Vidall C, Fernández-Ortega P, Cortinovis D, et al. Impact and management of chemotherapy/radiotherapy-induced nausea and vomiting and the perceptual gap between oncologists/oncology nurses and patients: a cross-sectional multinational survey. Support Care Cancer 2015;23:3297-305. [Crossref] [PubMed]
- Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 2004;100:2261-8. [Crossref] [PubMed]
- Roscoe JA, Heckler CE, Morrow GR, et al. Prevention of delayed nausea: a University of Rochester Cancer Center Community Clinical Oncology Program study of patients receiving chemotherapy. J Clin Oncol 2012;30:3389-95. [Crossref] [PubMed]
- Wang SY, Yang ZJ, Zhang L. Olanzapine for preventing nausea and vomiting induced by moderately and highly emetogenic chemotherapy. Asian Pac J Cancer Prev 2014;15:9587-92. [Crossref] [PubMed]
- Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011;9:188-95. [Crossref] [PubMed]
- Abe M, Hirashima Y, Kasamatsu Y, et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer 2016;24:675-82. [Crossref] [PubMed]
- Navari RM, Nagy CK, Gray SE. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 2013;21:1655-63. [Crossref] [PubMed]
- del Giglio A, Soares HP, Caparroz C, et al. Granisetron is equivalent to ondansetron for prophylaxis of chemotherapy-induced nausea and vomiting: results of a meta-analysis of randomized controlled trials. Cancer 2000;89:2301-8. [Crossref] [PubMed]
- Chiou TJ, Tzeng WF, Wang WS, et al. Comparison of the efficacy and safety of oral granisetron plus dexamethasone with intravenous ondansetron plus dexamethasone to control nausea and vomiting induced by moderate/severe emetogenic chemotherapy. Zhonghua Yi Xue Za Zhi (Taipei) 2000;63:729-36. [PubMed]
- de Wit R, de Boer AC. Effective cross-over to granisetron after failure to ondansetron, a randomized double blind study in patients failing ondansetron plus dexamethasone during the first 24 hours following highly emetogenic chemotherapy. Br J Cancer 2001;85:1099-101. [Crossref] [PubMed]