Should dexamethasone be standard in the prophylaxis of pain flare after palliative radiotherapy for bone metastases?—a debate
Pro argument
The use of palliative radiotherapy with either single or multiple fraction regimens has been shown to be an effective treatment for symptomatic bone metastases (1). However, a temporary worsening of pain after treatment (pain flare) can be a common in-field side effect (2-14) and has been reported in up to 68% of patients in some series (10), as shown in Table 1. Certainly, this represents a significant clinical problem for patients seeking symptomatic relief with radiotherapy. As a result, Symptom Control 23 (SC.23), a double-blind, randomized placebo-controlled trial was completed in order to answer the question: “Does dexamethasone provide prophylaxis for radiation-induced pain flare after palliative radiotherapy for bone metastases?” Building on a careful review of the available literature, an evidence-based argument for the use of prophylactic dexamethasone to reduce the incidence and severity of pain flare is presented below.
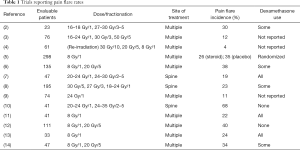
Full table
A prospective randomized trial investigating the prophylactic use of dexamethasone in this setting was recently published by Chow et al. in 2015 (5). Out of 298 patients undergoing a single fraction of 8 Gy to symptomatic bone metastasis, 148 patients were randomized to dexamethasone (8 mg orally for 5 days, starting the day of radiotherapy), and 150 patients to the placebo arm. This showed a 25% relative risk reduction of pain flare in the dexamethasone group, which equated to a number needed to treat (NNT) of 11. To put this into context, the NNT with aspirin to prevent death in patients with previously known heart disease or stroke is 333 (15). Additionally, quality of life scores in nausea, functional interference and appetite were improved in the dexamethasone arm. Considering these findings, a measurable improvement was readily achievable with this simple intervention.
Opponents of dexamethasone use often site the risk of toxicities such as hyperglycemia and GI upset. In this study (5), only three patients developed hyperglycemia, all of which were managed in the outpatient setting. The rates of grade 1–2 nausea were the same between both groups. The low toxicity of short courses of dexamethasone is mirrored in the post-operative pain literature. In a review of 5,796 patients receiving 1.25–20 mg of dexamethasone post-operatively, there was no increase in infection or delayed wound healing, and blood glucose levels were only 0.39 mmol/liter higher at 24 hours on average (16). Given the balance of benefits and risks of prophylactic dexamethasone, the authors of SC.23 came to the conclusion that “prophylactic use of dexamethasone should be adopted as standard of care for patients receiving palliative radiotherapy for treatment of painful bone metastases.”.
These results showing an improvement in pain flare rate with prophylactic dexamethasone have also been mirrored in other studies. In the spine stereotactic body radiotherapy (SBRT) literature, Khan et al. performed a prospective observational study of 47 patients undergoing spine SBRT (7). Twenty-four patients received 4 mg of dexamethasone for 5 days, and 23 patients were given 8 mg of dexamethasone for 5 days. Only 19.2% of patients experienced pain flare. This was a substantial improvement when compared to data from the same institution which reported a 68% incidence of pain flare in steroid naïve patients undergoing spine SBRT (10).
Also important when justifying the use of a prophylactic medication is having a biologic rationale for its use. As reviewed in detail by Barnes in the British Journal of Pharmacology, corticosteroids are strong anti-inflammatory agents working through several distinct biologic pathways (17). Though the exact biologic mechanism is not yet known for the action of dexamethasone in the prophylactic treatment of pain flare, some promising early data has been published. Bushehri et al. analyzed 83 urine samples from 46 patients receiving a single 8 Gy for painful bone metastases. Overall, differences were detected in levels of several pro-inflammatory urinary cytokines and chemokines when comparing those who had pain flare and those who did not (18). These early observations, though limited by small numbers, do provide some circumstantial evidence supporting the biological plausibility for the use of strong anti-inflammatory agents in this setting. Further study into the exact inflammatory pathways leading to pain flare is needed before the true mechanism of dexamethasone function in this setting can be ascertained.
Another important factor to consider for patients receiving palliative radiotherapy is the limited life expectancy of patients with bone metastases. Many patients with bone metastases have a median survival of 7–9 months (1). Therefore, a significant increase in pain for even a few days could potentially represent a significant proportion of a patient’s remaining lifespan. In the setting of limited lifespan, the importance of even small improvements in other quality of life measures (as previously discussed in the context of SC.23) becomes even more clinically relevant.
In summary, available randomized data supports the benefit of prophylactic dexamethasone in reducing pain flare in patients receiving palliative radiotherapy for painful bone metastases. Also significant is the relatively low risk of toxicities associated with a short course of steroids, and other beneficial effects of steroids, such as improved quality of life measures (i.e., nausea, functional impairment and appetite). In this patient population, a simple intervention with prophylactic dexamethasone can make a significant difference in symptom management and therefore should be standard for all patients undergoing palliative radiotherapy for painful bone metastases.
Con argument
Pain flare is a well-documented side-effect of palliative radiotherapy for painful bone metastases. Including the SC.23 trial, there have been multiple prospective studies showing pain flare rates between 11% and 68%. While the SC.23 trial showed a statistically significant reduction in pain flare incidence with the use of prophylactic dexamethasone, the clinical impact of this intervention must be carefully addressed before dexamethasone can be adopted as the standard of care. Indeed, though a statistically significant reduction in pain flare of 9% was observed in this trial, more than a quarter of patients continued to experience this toxicity. Both the efficacy and safety profile of the proposed intervention must be considered.
In SC.23, the median increase in pain score was 2 points with a range of 0–6, and the average duration of the pain flare was 3 days in the dexamethasone arm and 2 days in the placebo arm (5). The detailed quality of life outcomes from the SC.23 trial are pending. However previous prospective studies do not show any deterioration in global quality of life after pain flare at 6 weeks (11).
The survival of patients with metastatic cancer receiving palliative radiotherapy is steadily improving in the last decade across most tumor sites (19). Patients with favorable disease biology and available therapies may survive for many years with metastatic disease (20). Therefore, a median 2 point increase in worst pain score for 2–3 days may not be clinically significant and previous studies investigating the minimally clinically important differences in the brief pain inventory show that a 2 point difference is borderline significant (20).
In addition to the minimal clinical impact of pain flare attenuation, the mechanism of action for dexamethasone in pain flare prophylaxis remains unclear. It has been postulated that pain flare may be mediated by the release of inflammatory cytokines (21). However it is unclear if dexamethasone acts as an anti-inflammatory agent or as an analgesic, or a combination of both effects. There is evidence to suggest that dexamethasone may have a moderate analgesic effect in cancer pain (22). It is interesting to note that there was no difference in pain flare between the two groups at days 6–10. This result partially supports the effect of dexamethasone as an analgesic, because once the dexamethasone was stopped (day 5), there was no prophylactic effect anymore. If the mechanism of dexamethasone is better understood, other rational contenders could also be tested against dexamethasone. It should be noted that the study excluded patients on nonsteroidal anti-inflammatory drugs, which have both an anti-inflammatory and analgesic effect, similar to dexamethasone.
Also, could opioids have been better titrated better in anticipation of pain flare? There was no difference in median analgesic consumption between the two arms and between days 0–5 and 6–10, despite the differences in pain scores. Strong opioids are very efficient, having a NNT of 2–3 (23) whereas dexamethasone was shown to have a NNT of 9–11 in SC.23.
The other stated benefits of dexamethasone in the study were reduced nausea and increased appetite. For nausea, there are a number of potent antiemetic agents in other classes such as 5HT3 antagonists and dopamine antagonists. It is unclear whether patients received appropriate nausea prophylaxis/treatment in the study. It is known that patients receiving palliative radiotherapy can experience radiation-induced nausea and vomiting, and the use of anti-emetic treatment amongst radiation oncologists can vary significantly (24). Regarding the observed change in appetite, there was no improvement in the EORTC QLQ-C15-PAL instrument, which assesses change in patients who lack appetite. The questionnaire specifically asks “Have you lacked appetite?” However there was a difference in the appetite item of the dexamethasone symptom questionnaire (DSQ), which is designed to assess the side effects from dexamethasone. In the DSQ, the question about appetite is framed differently: “Have you had increased appetite?” Therefore patients who have a normal appetite could have a pathologic increase in appetite and it can be argued that the DSQ appetite score difference between the two arms is in fact a toxicity of dexamethasone, not a benefit.
In addition to increased appetite, there are concerns with other toxicities resulting from glucocorticoid therapy. While most of the adverse effects such as diabetes, peptic ulcer disease and osteoporosis usually result from chronic use, even a short course of glucocorticoids can cause side effects that can significantly impair quality of life. In SC.23 at day 42, significantly more patients receiving dexamethasone had depression compared to the placebo arm (8% vs. 1%, P=0.04). Indeed, mood and psychiatric disturbances are a well-established side effect of glucocorticoid therapy, even after short courses of treatment (25).
In summary, despite the results of a well-designed randomized trial showing the efficacy of dexamethasone in reducing the incidence of pain flare, further confirmatory trials are required and more questions need to be answered before dexamethasone can be adopted as the standard of treatment for pain flare prophylaxis in all patients. Several questions which arise include the following:
- Can a high-risk cohort of patients be identified to benefit from dexamethasone, in order to minimize the NNT and maximize the treatment benefit? Would patients who previously had a pain flare with palliative radiotherapy be better suited to this intervention?
- What is the biological mechanism of dexamethasone in preventing pain flare? Is it an alternative analgesic, an anti-inflammatory agent or a combination of both?
- Are there other agents that can be used as substitutes with a better therapeutic ratio and avoidance of the neuropsychiatric effects of dexamethasone?
Conclusions
Although the SC.23 trial showed that dexamethasone reduced the incidence of pain flare, consensus was not reached regarding the routine use of dexamethasone for all patients with bone metastases to be treated with radiation. The decision to use dexamethasone for the prophylaxis of radiation induced pain flare should be individualized and should take into account the risk of pain flare (e.g., higher risk with spine SBRT) and patient comorbidities (e.g., those with medical contraindications to steroids such as uncontrolled diabetes, uncontrolled hypertension, active peptic ulcer, hypokalemia and hyperglycemia).
The results of a randomized trial from the Netherlands, also investigating the use of dexamethasone in the prophylactic treatment of radiation induced pain flare, are currently pending, and may provide further guidance for physicians and patients in this setting (26).
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Edward Chow was the principal investigator of the SC.23 trial and the lead author on the manuscript reporting the results (5). All authors are affiliated with the University of Toronto.
References
- Chow E, Zeng L, Salvo N, et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112-24. [Crossref] [PubMed]
- Bernstein MB, Chang EL, Amini B, et al. Spine Stereotactic Radiosurgery for Patients with Metastatic Thyroid Cancer: Secondary Analysis of Phase I/II Trials. Thyroid 2016;26:1269-75. [Crossref] [PubMed]
- Muldermans JL, Romak LB, Kwon ED, et al. Stereotactic Body Radiation Therapy for Oligometastatic Prostate Cancer. Int J Radiat Oncol Biol Phys 2016;95:696-702. [Crossref] [PubMed]
- Hirano Y, Nakamura N, Zenda S, et al. Incidence and severity of adverse events associated with re-irradiation for spine or pelvic bone metastases. Int J Clin Oncol 2016;21:609-14. [Crossref] [PubMed]
- Chow E, Meyer RM, Ding K, et al. Dexamethasone in the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases: a double-blind, randomised placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:1463-72. [Crossref] [PubMed]
- Gomez-Iturriaga A, Cacicedo J, Navarro A, et al. Incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: multicenter prospective observational study. BMC Palliat Care 2015;14:48. [Crossref] [PubMed]
- Khan L, Chiang A, Zhang L, et al. Prophylactic dexamethasone effectively reduces the incidence of pain flare following spine stereotactic body radiotherapy (SBRT): a prospective observational study. Support Care Cancer 2015;23:2937-43. [Crossref] [PubMed]
- Pan HY, Allen PK, Wang XS, et al. Incidence and predictive factors of pain flare after spine stereotactic body radiation therapy: secondary analysis of phase 1/2 trials. Int J Radiat Oncol Biol Phys 2014;90:870-6. [Crossref] [PubMed]
- Owen D, Laack NN, Mayo CS, et al. Outcomes and toxicities of stereotactic body radiation therapy for non-spine bone oligometastases. Pract Radiat Oncol 2014;4:e143-9. [Crossref] [PubMed]
- Chiang A, Zeng L, Zhang L, et al. Pain flare is a common adverse event in steroid-naïve patients after spine stereotactic body radiation therapy: a prospective clinical trial. Int J Radiat Oncol Biol Phys 2013;86:638-42. [Crossref] [PubMed]
- Hird A, Zhang L, Holt T, et al. Dexamethasone for the prophylaxis of radiation-induced pain flare after palliative radiotherapy for symptomatic bone metastases: a phase II study. Clin Oncol (R Coll Radiol) 2009;21:329-35. [Crossref] [PubMed]
- Hird A, Chow E, Zhang L, et al. Determining the incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: results from three canadian cancer centers. Int J Radiat Oncol Biol Phys 2009;75:193-7. [Crossref] [PubMed]
- Chow E, Loblaw A, Harris K, et al. Dexamethasone for the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases: a pilot study. Support Care Cancer 2007;15:643-7. [Crossref] [PubMed]
- Loblaw DA, Wu JS, Kirkbride P, et al. Pain flare in patients with bone metastases after palliative radiotherapy--a nested randomized control trial. Support Care Cancer 2007;15:451-5. [Crossref] [PubMed]
- Antithrombotic Trialists' (ATT) Collaboration, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60. [Crossref] [PubMed]
- Waldron NH, Jones CA, Gan TJ, et al. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth 2013;110:191-200. [Crossref] [PubMed]
- Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol 2006;148:245-54. [Crossref] [PubMed]
- Bushehri A, Chow E, Zhang L, et al. Urinary cytokines/chemokines pattern in patients with painful bone metastases undergoing external beam radiotherapy experiencing pain flare. Ann Palliat Med 2016;5:107-15. [Crossref] [PubMed]
- McDonald R, Zhang L, Bedard G, et al. Change in overall survival associated with Karnofsky Performance Status over time. ResearchGate 2015;8:123-31.
- Mease PJ, Spaeth M, Clauw DJ, et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res (Hoboken) 2011;63:821-6. [Crossref] [PubMed]
- Svendsen KB, Andersen S, Arnason S, et al. Breakthrough pain in malignant and non-malignant diseases: a review of prevalence, characteristics and mechanisms. Eur J Pain 2005;9:195-206. [Crossref] [PubMed]
- Paulsen Ø, Aass N, Kaasa S, et al. Do corticosteroids provide analgesic effects in cancer patients? A systematic literature review. J Pain Symptom Manage 2013;46:96-105. [Crossref] [PubMed]
- Oxberry SG, Simpson KH. Pharmacotherapy for cancer pain. Contin Educ Anaesth Crit Care Pain 2005;5:203-6. [Crossref]
- Dennis K, Zhang L, Lutz S, et al. International patterns of practice in the management of radiation therapy-induced nausea and vomiting. Int J Radiat Oncol Biol Phys 2012;84:e49-60. [Crossref] [PubMed]
- Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days' corticosteroid treatment. A prospective study. Psychoneuroendocrinology 1996;21:25-31. [Crossref] [PubMed]
- Westhoff PG, de Graeff A, Geerling JI, et al. Dexamethasone for the prevention of a pain flare after palliative radiotherapy for painful bone metastases: a multicenter double-blind placebo-controlled randomized trial. BMC Cancer 2014;14:347. [Crossref] [PubMed]


