Plasma L-carnitine levels in terminally ill cancer patients receiving only palliative care
Introduction
Cancer-related fatigue and/or cachexia are common problems in cancer patients. Although several factors contributing to cancer-related fatigue and cachexia have been proposed, decreases in food intake combined with decreases in physical exercise lead to declines in both muscle mass and power. Anorexia and nausea exacerbate the detrimental effects of tumor-related changes in protein metabolism and nutritional status, leading to deterioration of the quality of life (QOL) in cancer patients (1,2). These metabolic disorders are associated with increases in local and systemic inflammatory mediators, such as proinflammatory cytokines. Thus, the pathogenesis of cancer-related fatigue and cachexia is multifactorial and complex (1,2).
L-carnitine (LC) is a natural endogenous cofactor for the translocation of long-chain fatty acids from the cytoplasmic compartment into the mitochondria, where beta-oxidation enzymes are located (3). LC deficiency has been found in certain cancer patients and is associated with fatigue (4-8). Thus, LC supplementation in cancer patients was suggested as a therapeutic option for cancer-related fatigue (9-11). However, LC supplementation did not improve fatigue in patients with invasive malignancies in a large, randomized, double blind clinical trial in non-selected cancer patients with good performance status (PS) (11). In contrast, recent studies performed in selected patients treated with chemotherapy or chemoradiotherapy indicated that supplementation with LC increased plasma LC levels and decreased weight loss, increased BMI, and improved both nutritional status and QOL in pancreatic cancer patients (12) or breast cancer (13) compared with placebo controls. Thus, a better understanding of LC in selected cancer patients may lead to increased treatment possibilities in patients with malignancies.
The few studies that have evaluated plasma LC status and the potential association with fatigue in terminally ill cancer patients have been reported, especially in Japanese patients. Therefore, in the present study, we focused on the plasma LC levels in terminally ill cancer patients treated with palliative care with expected survival of less than 2 months. In addition, we examined the relationships with laboratory parameters, including albumin, C-reactive protein (CRP), etc.
Methods
Study protocol and subjects
The protocol for this prospective study was approved by the Institutional Ethical Committee of Shinshu University School of Medicine, Matsumoto, Japan (document number: 2783) on July 8, 2014. The study population consisted of a series of consecutive cancer patients referred to the palliative care team of our hospital from April 2015 to March 2016. We evaluated palliative prognosis index (PPI) score (14) at the first meeting with the palliative care team, and patients with PPI >2.0 were enrolled in the present study. Patients under 20 years old were excluded from the present study. As a control group, advanced and metastatic chemo-naive cancer patients scheduled to receive cisplatin-containing chemotherapy as first-line therapy were selected to compare LC levels. Written informed consent was obtained from each patient enrolled in this study. Calculation of sample size was performed using G*power 3.1.0 software (15), assuming α error =0.05, power =80%, and estimated effect size =0.8. Twenty-one subjects were needed for each group.
Blood samples
We determined not only plasma LC levels but routine chemistry, including albumin, C-reactive protein (CRP), aspartate aminotransferase, alanine aminotransferase, creatinine, and total bilirubin. These blood data were examined in the morning between 07:00 and 09:00 after an overnight fast. Venous blood samples were taken and stored in tubes containing ethylenediaminetetraacetic acid (EDTA). In the control group, blood samples were obtained before cisplatin administration. LC levels in all specimens were evaluated by SRL Inc. (Tokyo, Japan). Total and free LC levels were measured by an enzyme cycling method using an autoanalyzer (JCA-BM8000 series; JEOL, Tokyo, Japan) and acyl-LC was calculated as the difference between total and free LC (16).
Statistical analysis
The results are presented as means ± standard deviation. The two-tailed Mann-Whitney U-test was used at the P=0.05 level of significance. All statistical analyses were performed with GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA). The actual overall survival after enrollment was calculated using the Kaplan-Meier method and differences in the resulting distributions were compared by the log-rank test.
Results
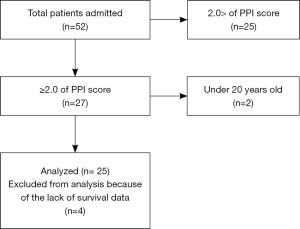
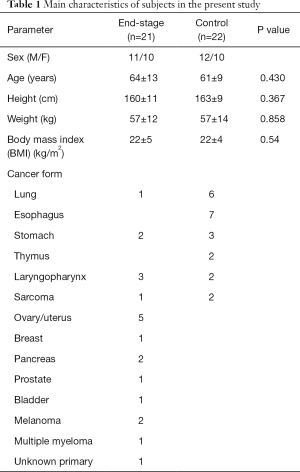
Fifty-two patients were referred to the palliative care team, and the PPI scores of 27 patients were >2.0, two of whom were <20 years old. Therefore, 25 patients were initially included in the analysis, but four were excluded because of lack of survival data (Figure 1). Twenty-two chemo-naive cancer patients were evaluated as a control group. Baseline characteristics in terminally ill (end-stage) and control subjects are shown in Table 1. There were no significant differences in terms of age, height, or weight between the two groups. Various malignant diseases were included in end-stage palliative patients. Diagnoses in control group were as follows: lung cancer, 6; esophageal cancer, 7; stomach cancer, 3; thymic cancer, 2; laryngopharyngeal cancer, 2; and sarcoma, 2. The detailed characteristics regarding PPI score, median survival time, and weight change for 3 months before obtaining blood samples in terminally ill patients are summarized in Table 2. The median PPI score was 5.5 (range, 2–13.5), median survival time was 38.5 days (range, 1–90 days), and mean weight change was –0.4±7.3 kg.
Full table

Full table
Comparisons of laboratory parameters between terminally ill and control patients are summarized in Table 3. In the terminally ill patients, mean albumin and CRP levels were 2.7±0.5 g/dL and 8.3±6.0 mg/mL, respectively, whereas corresponding values in the control group were 3.9±0.4 g/dL and 0.5±1.0 mg/dL, respectively. There are differences in albumin and CRP levels between the two groups were significant (P<0.001). There were no significant differences in other parameters, including hepatic and renal functions between the two groups.

Full table
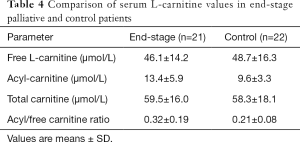
The total, free, and acyl-LC levels in both groups are shown in Table 4; their plasma concentrations in terminally ill cancer patients were 59.5±16.0, 46.1±14.2, and 13.4±5.9 µmol/L, respectively. These values were not significantly different from those in the control group of chemo-naive patients (58.3±18.1, 48.7±16.3, and 9.6±3.3 µmol/L, respectively).
Full table
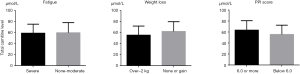
We estimated the LC levels in terminally ill patients according to the clinical parameters. We analyzed the correlations between LC level and albumin or CRP levels in each subject. However, neither albumin nor CRP was correlated with LC level (data not shown). In addition, 10 patients (47.6%) complained of severe fatigue and nine (42.9%) showed weight loss over –2 kg over the 2 months prior to the examination. LC levels were compared with and without symptoms. Furthermore, we compared the levels of LCs between subgroups divided according to PPI score above and below 6.0. No significant differences were detected between these two subgroups (Figure 2). Median survival time in patients with PPI score >6.0 was significantly lower than that in patients with PPI <6.0 (23.5 vs. 55.5 days, respectively, P<0.001).
Discussion
We examined the plasma LC levels in terminally ill cancer patients with expected survival of several months treated with only palliative care in Japanese hospital. We found that plasma LC levels remained normal and were independent of the presence of general fatigue. In addition, the levels were not associated with plasma albumin and CRP levels in these patients.
Cachexia is a serious and common problem in cancer management, especially in terminally ill cancer patients (1,2). As the biological role of LC is related to energy metabolism, LC level was speculated to be involved in general deterioration in our subjects expected to survive for only a few months. However, the values of plasma total, free, and acyl-LC were almost normal and LC deficiency (total LC <35 µmol/mL) was only observed in 5% of patients included in the present study. Vinci et al. (8) reported significantly lower serum LC levels in patients with tumor cachexia compared to normal controls. The value of total LC was 43.9 µmol/L, which was lower than that in the present study. Based on the randomized, double blind, placebo-control Phase III trial of LC supplementation performed by Cruciani et al. (9), LC deficiency was observed in 33% of the enrolled subjects before supplementation. Thus, although our study included various malignancies and a clinically heterogeneous population, the present study indicated that plasma LC status may be independent in terminally ill and hospitalized Japanese patients with expected survival within 2 months.
It was noteworthy that plasma LC level was independent of clinical factors, such as the presence of fatigue or weight loss. The results suggested that LC level is unrelated to these clinical parameters. In addition, plasma LC levels were also not associated with CRP or albumin levels. CRP and albumin levels have been used to assess the prognosis of several cancers as the Glasgow prognostic score (17). In addition, we analyzed LC levels according to subgroup, and found that LC level in patients with high CRP (>1.0 mg/mg) plus hypoalbuminemia (<3.5 mg/dL) was not lower compared with other groups in the present study (data not shown). Thus, our study indicated that plasma LC status was independent of serum CRP and albumin values in terminally ill cancer patients.
The present study had several limitations. First, patients in our study had many different types of malignancy. The main evaluation in the present study was to determine plasma LC status in terminally ill patients in Japan, but the numbers of patients with each type of malignancy were too small to allow conclusions to be drawn with respect to potential differences in LC levels between the different malignancies. Second, we included clinically heterogeneous patients, e.g., with various levels of food intake, etc. In addition, the present study included various types of malignancy and heterogeneous patients, such as those with increases in body weight because of systemic edema or pleural and peritoneal effusion. In addition, most patients were treated with minimal fluid therapy in the present study because of their hospitalization.
In summary, we demonstrated that LC level in plasma remained normal even in terminally ill and hospitalized cancer patients in Japan. Our results suggested that LC deficiency is not always common even in terminally ill and hospitalized cancer patients and is independent of chemical and clinical parameters, such as albumin, CRP, fatigue, and weight loss.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol for this prospective study was approved by the Institutional Ethical Committee of Shinshu University School of Medicine, Matsumoto, Japan (document number: 2783) on July 8, 2014.
References
- Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin 2002;52:72-91. [Crossref] [PubMed]
- Argilés JM, López-Soriano FJ, Busquets S. Mechanisms and treatment of cancer cachexia. Nutr Metab Cardiovasc Dis 2013;23 Suppl 1:S19-24. [Crossref] [PubMed]
- Rebouche CJ. Quantitative estimation of absorption and degradation of a carnitine supplement by human adults. Metabolism 1991;40:1305-10. [Crossref] [PubMed]
- Silvério R, Laviano A, Rossi Fanelli F, et al. l-carnitine and cancer cachexia: Clinical and experimental aspects. J Cachexia Sarcopenia Muscle 2011;2:37-44. [Crossref] [PubMed]
- Hockenberry MJ, Hooke MC, Gregurich M, et al. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J Pediatr Hematol Oncol 2009;31:664-9. [Crossref] [PubMed]
- Endo K, Tsuji A, Kondo S, et al. Carnitine is associated with fatigue following chemoradiotherapy for head and neck cancer. Acta Otolaryngol 2015;135:846-52. [Crossref] [PubMed]
- Malaguarnera M, Risino C, Gargante MP, et al. Decrease of serum carnitine levels in patients with or without gastrointestinal cancer cachexia. World J Gastroenterol 2006;12:4541-5. [Crossref] [PubMed]
- Vinci E, Rampello E, Zanoli L, et al. Serum carnitine levels in patients with tumoral cachexia. Eur J Intern Med 2005;16:419-23. [Crossref] [PubMed]
- Cruciani RA, Dvorkin E, Homel P, et al. L-carnitine supplementation for the treatment of fatigue and depressed mood in cancer patients with carnitine deficiency: a preliminary analysis. Ann NY Acad Sci 2004;1033:168-76. [Crossref] [PubMed]
- Mantovani G, Macciò A, Madeddu C, et al. Randomized phase III clinical trial of five different arms of treatment for patients with cancer cachexia: interim results. Nutrition 2008;24:305-13. [Crossref] [PubMed]
- Cruciani RA, Zhang JJ, Manola J, et al. L-carnitine supplementation for the management of fatigue in patients with cancer: an eastern cooperative oncology group phase III, randomized, double-blind, placebo-controlled trial. J Clin Oncol 2012;30:3864-9. [Crossref] [PubMed]
- Kraft M, Kraft K, Gärtner S, et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN) - a randomized multicentre trial. Nutr J 2012;11:52. [Crossref] [PubMed]
- Iwase S, Kawaguchi T, Yotsumoto D, et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and L-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: a multi-institutional, randomized, exploratory trial (JORTC-CAM01). Support Care Cancer 2016;24:637-46. [Crossref] [PubMed]
- Yamada T, Morita T, Maeda I, et al. A prospective, multicenter cohort study to validate a simple performance status-based survival prediction system for oncologists. Cancer 2017;123:1442-52. [Crossref] [PubMed]
- Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175-91. [Crossref] [PubMed]
- Takahashi M, Ueda S, Misaki H, et al. Carnitine determination by an enzymatic cycling method with carnitine dehydrogenase. Clin Chem 1994;40:817-21. [PubMed]
- Jeong JH, Lim SM, Yun JY, et al. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology 2012;83:292-9. [Crossref] [PubMed]