Covered biliary stents with proximal bare stent extension for the palliation of malignant biliary disease: can we reduce tumour overgrowth rate?
Introduction
Despite the significant advances of medical imaging and preventive medicine, even nowadays, the majority of malignant biliary strictures cases are detected in an advanced stage and there is no curative resection option. Nevertheless, such patients may be in a good general status and if metastatic spread is controlled by chemotherapy they may benefit from a reasonable period of survival. Symptomatic relief is of paramount importance for quality of life of such patients with first line target the effective and long-term management of jaundice that is achieved with the use of self-expandable metallic biliary stents. Bare stents however tend to get occluded from tumour ingrowth; covered stents have been developed and are now the first line treatment offering better results regarding patency and re-intervention rate (1-4).
Covered stents on the other hand cannot be deployed everywhere in the biliary tree due to anatomical restrictions (i.e., bile duct bifurcation, cystic duct orifice) and their use is mainly limited in the common bile duct strictures. Furthermore, dysfunction may occur even with the use of covered stents and the main cause is growth of tumour proximally and distally to the stent’s ends (tumour overgrowth) where there is no covering membrane. Less frequently, covered stent dysfunction may occur due other causes like sludge formation or distal stent migration (5,6). Whereas blockage from sludge may easily be treated with the use of a balloon catheter and distal migration may be prevented with the use of stent anchoring fins, prevention of tumour overgrowth still remains an unsolved issue in the management of such patients (4,5,7).
Purpose of this study is to assess the combination of a covered stent and a bare extension in the palliative treatment of patients with inoperable biliary disease due to a Bismuth type I/II lesion and life expectancy longer than 6 months.
Methods
Study design and patients
This is a prospective, single arm, single centre, cohort study. Study hypothesis is that the use a covered biliary stent with an uncovered extension, would prevent from stent’s occlusion from both tumour ingrowth and overgrowth. The composite endpoint was defined as patient death or endoprosthesis occlusion. The study was approved by the institutional review board (IRB) (number of approval document 127/2011) and applied according to the guidelines described in the Declaration of Helsinki for biomedical research involving human subjects. Written informed consent was obtained from the patients for publication. Physicians with considerable experience on percutaneous biliary interventions performed the procedures.
Inclusion criteria were: (I) obstructive jaundice caused by unresectable Bismuth type I or II lesion that has been considered inoperable from a regional multidisciplinary meeting; (II) performance status >3 on the Eastern Cooperative Oncology Group scale (8); (III) absence of distal metastases other than to the adjacent lymph nodes; (IV) absence of cirrhosis with portal hypertension. Exclusion criteria were: (I) age older than 80 years; (II) previous surgical or radiotherapy palliative treatment; (III) gastric outlet obstruction. The presence of ascites was not considered a contraindication and in these patients a left side approach was chosen. Prior to the procedure, a written informed consent was obtained from all patients.
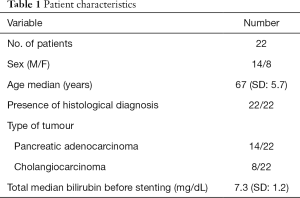
Twenty-two patients were included in the study. They were 14 men and 8 women, with age range of 61–78 years (mean 69.2, median 67 years). Tumour diagnosis was based on histology after forceps biopsy or fine needle aspiration. Patients’ characteristics are described in Table 1.
Full table
In all patients an ePTFE/FEP (expanded polytetrafluoroethylene/fluorinated ethylene propylene) self-expandable covered stent with anchoring fins (Viabil, Gore, Flagstaff, AZ, USA) was deployed with a self-expandable woven stainless steel alloy uncovered proximal and distal extension (Wallstent and Wallflex, Boston Scientific, Natick, MA, USA). The covered stent was deployed first and then the bare stent was deployed telescopically, inside the lumen of the covered stent.
Materials and methods
Pre-procedural imaging with computed tomography (CT) or magnetic resonance imaging (MRI) was performed in order to exclude any metastatic deposits in the hepatic parenchyma, to assess the level of biliary tree dilatation to exclude portal vein thrombosis.
Patients were kept fasted for 6 hours prior to the procedure and premedication with 750 mg of Cefuroxime was given. The procedure was performed under conscious sedation with Midazolam (1 to 5 mg) and Fentanyl (25 to 100 µg).
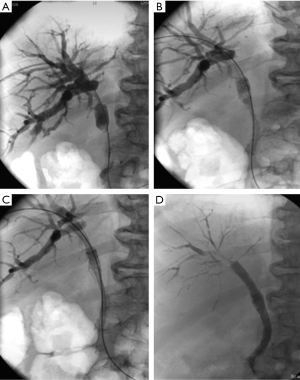
Ultrasound (US) or fluoroscopically guided access to the biliary tree was obtained. In the cases where no definitive decision from the multidisciplinary meeting was made and external drain was inserted to relief from jaundice. In case no biopsy was present a biopsy specimen was obtained with the use of endobiliary forceps. When a decision for stenting was made the stricture was crossed and a cholangiogram with the use of a measuring pigtail catheter was performed to delineate the length of the stricture. The covered stent was then deployed in the common bile duct. When the cystic duct was patent the covered stent was placed with the proximal end of at the level of the cystic duct. In the cases where the cystic duct was already infiltrated by tumour or where previous cholecystectomy was performed the covered stent was deployed until the mid-level of the common hepatic duct. The bare stent was deployed in a way that approximately one centimetre was protruding distally to the distal end of the covered stent and approximately two centimetres proximally to the proximal end. In case of tight strictures, pre-dilatation and post-dilatation with a balloon catheter was performed. If no bleeding occurred the hepatic tract was plugged with gel foam pellets otherwise or an access catheter was left in situ and for 1–2 days (Figure 1).
Follow-up and re-intervention
Follow-up parameters consisted in blood laboratory exams, clinical findings and imaging on an outpatient basis. When the patient was presented with jaundice or cholangitis, stent occlusion was suspected. Imaging (US or CT) and clinical evaluation confirmed stent occlusion and re-intervention with Percutaneous transhepatic cholangiogram (PTC) followed.
Study end points and definitions
Primary study endpoints were the assessment of technical success, accuracy and safety of uncovered extension deployment through the covered stent, stent patency and overall patient survival. Secondary study endpoints were the characterization of the type of stent dysfunction.
Technical success was defined as the deployment of the covered endoprosthesis at the level of the stricture and of the uncovered one with the proximal end at least two cm proximally to the proximal end of the covered stent and the distal end at least one cm distal to the distal end of the covered one. The deployment in the stenotic area was expected to result to a residual lumen stenosis that was less than 30% of the initial lumen. Safety of stent deployment was correlated to the incidence of peri-procedural and intra-procedural complications. Complications were considered early if occurred in the first 30 days after the procedure, otherwise they were considered late and were classified according to the North American Society of Interventional Radiology (SIR) criteria (9).
Stent patency was defined as the absence of recurrent symptomatic biliary obstruction. Primary patency was defined as the interval time between initial placement and recurrence of obstruction. If there was no evidence of obstruction, during patient’s life, patency period was considered equal to the survival period, but censored.
Statistical analysis
Cumulative stent patency and patient’s survival were estimated by the Kaplan-Meier technique (10). Statistical analysis was done with NCSS 97 statistical package (NCSS Statistical Software, Salt Lake City, UT, USA).
Results
Ultrasound guidance was performed in 10/22 cases. In the remaining cases fluoroscopic guidance was used. A left side access was used in 3 cases. In 12/22 cases no access catheter was left after stenting and the tract was plugged with gelfoam pellets. In 8/22 cases a 5 Fr access catheter was left in situ and in 2/22 an 8 Fr internal-external drain catheter. The access catheter and drain were removed after a mean time of 4.1 days. A total number of 22 covered stents (10 mm wide and 4 or 6 cm long) and 22 bare stents (10 mm wide and 6.9 or 9 cm long) were used.
Technical success and complications
Technical success rate was 100%. All stents were successfully inserted and deployed where planned, and no case of stent migration occurred during deployment. Early complications were observed in two (9%) patients to whom moderate peritoneal irritation occurred during the procedure. Both patients were treated with pain control for 48 hours and the symptoms were resolved (class B according to SIR) (9). No late complications occurred.
Patency and survival
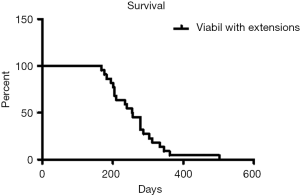
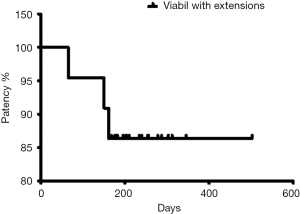
Patients were followed-up until death. No patient was lost during the follow-up period. The mean follow-up after stent placement was 263.7 days (range, 168–502 days). Thirty-day and 3-month mortality rate was zero. According to the Kaplan-Meier analysis, survival rates at 3, 6 and 12 months were 100%, 86% and 9%, respectively. Mean survival time was 263.7 days (median: 255, SD: 77.6) (Figure 2). Stent premature occlusion occurred in three cases. Mean patency was 240 days (median: 237, SD: 87). The primary patency rates at 3, 6 and 12 months were 90%, 86% and 86% respectively (Figure 3).
In the cases where endoprosthesis dysfunction occurred a new PTC was performed and an external biliary drain was initially placed. A biopsy specimen of the occlusion tissue was obtained and a semi-inflated balloon was used to “clean” the accumulated material. In all three cases dysfunction was attributed to sludge formation and not to tumour ingrowth. Balloon cleaning was effective in all three cases and new stenting was not required. In none of the cases tumour overgrowth was noted. Mean secondary patency was 221 days (median: 200, SD: 51.8).
Discussion
Palliation of malignant biliary disease is offered for the last twenty years with the use of self-expandable metallic stents (11). Bare metallic stents after their deployment in the bile ducts get covered by a thin epithelial layer, which after 2–4 months becomes thicker and is accompanied by the growth of tumour cells through the mesh of the stent, leading sooner or later to stent dysfunction (12).
Covered stents were developed to face the problem of tumour ingrowth. Several coverage materials were initially used with controversial results (13-18). The ePTFE/FEP coverage was combined with a nitinol skeleton in a stent with anchoring barbs and was used in four studies offering satisfactory results, with evidence of prevention of ingrowth and stent migration (19-22). In these four series stent’s dysfunction occurred mainly due to tumour overgrowth and sludge formation.
Prospective randomized studies have also shown that when covered stents are directly compared to uncovered ones, tumour overgrowth appears to be one of the main dysfunction causes among the covered stent groups. In the meta-analysis of Saleem et al. (4), involving 781 patients it is shown that the relative risk of tumour overgrowth in the covered stent groups was 2.03, with statistical significance between covered and uncovered stents (P=0.03). This result indicates that covered stents are able to control tumour ingrowth but not tumour overgrowth.
In order to limit tumour overgrowth a covered stent’s extension is required. In our study we followed this principle to merge the covered stent effect that prevents ingrowth with the extension effect that prevents tumour overgrowth. When an appropriate coverage membrane is used, like in this case the ePTFE/FEP, in a stent with anchoring barbs that would prevent migration then an uncovered extension is necessary in order to prevent from tumour overgrowth without occluding the cystic or the intrahepatic ducts. The uncovered extension might also be used distally, like we did in our case, offering a wide opening of the papillary area, which is necessary to prevent occlusion from sludge formation.
In our study we used a woven stainless steel alloy bare stent as an extension due to the given flexibility and radial force. The woven pattern of the stent offers a high radial force but when deployed in a stenotic area tends to increase in length and post-dilatation might be necessary in some cases.
The main limitations of the study are the retrospective nature and the low number of number of patients. Nevertheless, the purpose of the study is to investigate the device design that would offer longer patency in patients with malignant biliary disease, avoiding unnecessary re-interventions, hospitalization, cost and complications. We may conclude that the combined use of ePTFE/FEP covered stents and a woven stainless steel bare proximal and distal extension appear to offer an effective palliation measure for the use in patients with malignant biliary disease and a reasonable survival prediction. Further studies with novel stents that would incorporate both those design features are required in the future.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board (IRB) (number of approval document 127/2011) and applied according to the guidelines described in the Declaration of Helsinki for biomedical research involving human subjects. Written informed consent was obtained from the patients for publication.
References
- Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomized study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut 2004;53:729-34. [Crossref] [PubMed]
- Krokidis M, Fanelli F, Orgera G, et al. Percutaneous palliation of pancreatic head cancer: randomized comparison of ePTFE/FEP-covered versus uncovered nitinol biliary stents. Cardiovasc Intervent Radiol 2011;34:352-61. [Crossref] [PubMed]
- Krokidis M, Fanelli F, Orgera G, et al. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovasc Intervent Radiol 2010;33:97-106. [Crossref] [PubMed]
- Saleem A, Leggett CL, Murad MH, et al. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc 2011;74:321-327.e1. [Crossref] [PubMed]
- Telford JJ, Carr-Locke DL, Baron TH, et al. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc 2010;72:907-14. [Crossref] [PubMed]
- Kullman E, Frozanpor F, Söderlund C, et al. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc 2010;72:915-23. [Crossref] [PubMed]
- Lee MJ, Dawson SL, Mueller PR, et al. Failed metallic biliary stents: causes and management of delayed complications. Clin Radiol 1994;49:857-62. [Crossref] [PubMed]
- WHO handbook for reporting results of cancer treatment. Geneva: World Health Organization, 1979.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology Clinical Practice Guidelines. J Vasc Interv Radiol 2003;14:S199-202. [Crossref] [PubMed]
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958;53:457-81. [Crossref]
- Lammer J, Hausegger KA, Fluckiger F, et al. Common bile duct obstruction due to malignancy: Treatment with plastic versus metal stents. Radiology 1996;201:167-72. [Crossref] [PubMed]
- Boguth L, Tatalovic S, Antonucci F, et al. Malignant biliary obstruction: Clinical and histopathologic correlation after treatment with self-expanding metal prostheses. Radiology 1994;192:669-74. [Crossref] [PubMed]
- Rossi P, Bezzi M, Salvatori FM, et al. Clinical experience with covered Wallstents for biliary malignancies: 23-month follow-up. Cardiovasc Intervent Radiol 1997;20:441-7. [Crossref] [PubMed]
- Hausegger KA, Thurnher S, Bodendorfer G, et al. Treatment of malignant biliary obstruction with polyurethane covered Wallstents. AJR Am J Roentgenol 1998;170:403-8. [Crossref] [PubMed]
- Miyayama S, Matsui O, Terayama T, et al. Covered Gianturco stents for malignant biliary obstruction: preliminary clinical evaluation. J Vasc Interv Radiol 1997;8:641-8. [Crossref] [PubMed]
- Han YM, Jin GY, Lee S, et al. Flared Polyurethane-covered Self expandable Nitinol Stent for Malignant Biliary Obstruction. J Vasc Interv Radiol 2003;14:1291-301. [Crossref] [PubMed]
- Kanasaki S, Furukawa A, Kane T, et al. Polyurethane-covered nitinol Strecker stents as primary palliative treatment of malignant biliary obstruction. Cardiovasc Intervent Radiol 2000;23:114-20. [Crossref] [PubMed]
- Isayama H, Komatsu Y, Tsujino T, et al. Polyurethane-covered metal stent for management of distal malignant biliary obstruction. Gastrointest Endosc 2002;55:366-70. [Crossref] [PubMed]
- Schoder M, Rossi P, Uflacker R, et al. Malignant biliary obstruction: Treatment with ePTFE/FEP-covered endoprostheses-initial technical and clinical experiences in a multicenter trial. Radiology 2002;225:35-42. [Crossref] [PubMed]
- Bezzi M, Zolovkins A, Cantisani V, et al. New ePTFE/FEP-covered stent in the palliative treatment of malignant biliary obstruction. J Vasc Interv Radiol 2002;13:581-9. [Crossref] [PubMed]
- Hatzidakis A, Krokidis M, Kalbakis K, et al. ePTFE/FEP-covered metallic stents for palliation of malignant biliary disease: can tumor ingrowth be prevented? Cardiovasc Intervent Radiol 2007;30:950-8. [Crossref] [PubMed]
- Fanelli F, Orgera G, Bezzi M, et al. Management of malignant biliary obstruction: Technical and clinical results using an expanded polytetrafluoroethylene fluorinated ethylene propylene (ePTFE/FEP)-covered metallic stent after 6-year experience. Eur Radiol 2008;18:911-9. [Crossref] [PubMed]