Hospitalizations in elderly glioblastoma patients
Introduction
Approximately half of all patients diagnosed with glioblastoma (GB) are aged 65 years or older (1) and it is well known that the survival outcomes are worse for elderly patients with GB than for younger GB patients (2,3). A population-based study in the USA from the years 2000–2010 reported survival outcomes of 14,675 GB patients. In this study, the median overall survival among age groups was 29 months for ages 19–34, 18 months for ages 35–49, 13 months for ages 50–64, 8 months for ages 65–74 and 5 months for ages 75 and older (4).
Studies suggest that GB patients spend significant amounts of time in both acute and chronic care settings. Rahman et al. (5) found that the most common reason for hospital admission was weakness and immobility. Factors associated with hospitalization were older age and poorer performance status. Paszat and colleagues (6) reported in 2001 that 45% of patients with GB aged 60–69 years old spent at least half of their remaining survival after diagnosis as an inpatient with rates as high as 59% in 70–79 years old and 76% for those over 80 years of age. Out of 5,000 GB patients aged 65 and older, 21% were hospitalized for at least 30 cumulative days between diagnosis and death. Twenty-two percent of all patients spent at least one quarter of their remaining lives as an inpatient with the risk increasing with increasing age (1).
The management for patients age 65 and older for GB ranges from optimal supportive care alone, short course radiotherapy (1), temozolomide alone (7), short course radiotherapy with temozolomide chemotherapy (8), and protracted course radiotherapy (60 Gy in 30 daily fractions) with temozolomide chemotherapy (2). Management decisions are based on prognostic features such as age (65–70 years, 70 years and older), MGMT methylation status (9) and performance status (10). In general, patients who have poor performance status and poor survival, unlikely to benefit from treatment are considered for optimal supportive care alone. However, a randomized trial reported that for patients 70 years and older with anaplastic astrocytoma or GB patient and with Karnofsky performance status 70 or higher, radiation improves survival as compared to comfort measures (11). For patients age 65 years and older with Eastern Co-operative Oncology Group (ECOG) performance status 0–2, short course radiotherapy and temozolomide improves survival as compared to short course radiotherapy alone, based on a recently published trial (8). However, for patients who have excellent performance status in the lower end of the elderly age group (less than 70 years old), protracted radiation with temozolomide chemotherapy may be given based on the Stupp trial (2) which included patients up to the age of 70. For GB patients over the age of 70, either short course radiotherapy with (8) or without temozolomide or temozolomide alone may be considered (12). The use of temozolomide may be favoured in methylated MGMT GB (12).
Even with aggressive therapy (e.g., protracted radiation with temozolomide), survival for GB patients over the age of 65 is still short. The morbidity associated with GB and the potential toxicity of treatment puts this vulnerable group of patients at risk of hospitalizations.
The primary purpose of this study was to describe the proportion of survival spent in hospital in GB patients over the age of 65, initially managed at a tertiary cancer centre. The secondary objectives were to explore possible risk factors for admission and to explore costs.
Methods
Patients with newly diagnosed GB seen at a tertiary cancer centre from December 2006 to December 2014 were included. Demographic information (age at diagnosis, sex), baseline characteristics (performance status), treatment information (date of craniotomy, extent of surgical resection or biopsy, radiation dose fractionation, chemotherapy) and survival were linked anonymously with provincial administrative databases through the Institute of Clinical Evaluative Sciences (ICES).
The following sources and associated data were used:
- The Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD): dates of acute hospital admission;
- National Ambulatory Care Reporting System Database (NACRS): dates of care (includes emergency room visits, day procedures and cancer clinic visits) and resource utilization (based on resource intensity weights) (13). A resource intensity weight (RIW) is assigned to each hospital inpatient and represents the average amount of hospital resources (including administration, staff, supplies, technology and equipment) used by patients with a particular condition relative to the average resources used by other patients. For example, a patient with a RIW of 2.0 used twice as many resources as a patient with a RIW of 1.0;
- Ontario Health Insurance Plan (OHIP) Schedule of Benefits Claims Database: Physician services, date provided, fee paid. We used the palliative care fee codes (K023; C945; C882; C982; A902; A771; A945; W882; W982; W872; W972; G511; K700) as a surrogate for patient access to palliative care.
We determined patient costs for acute inpatient hospitalizations, ambulatory care, and physician services costs. Patient costs for acute hospitalizations (CIHI-DAD) and ambulatory care (NACRS) were calculated as the product of the resource weight, reflecting the intensity of service utilization for the specific episode and the appropriate unit cost (13).
Bivariate associations between baseline characteristics [age, sex, ECOG performance status, initial treatment type (biopsy, subtotal resection, gross total resection, chemotherapy, radiation, comfort measures only)] were identified using the chi-square and Fisher exact tests. Negative binomial regression modelling was performed using backward elimination of variables at P≥0.2, with the outcomes of interest being at least one visit to the emergency department, at least one admission to an inpatient acute care unit and length of stay. For the regression analyses, age and length of stay were treated as continuous variables. A P value of less than 0.05 was considered significant. All tests were 2-tailed and were performed using the SAS software application (version 9.2; SAS Institute, Cary, NC, USA).
The study was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre (Project Identification Number 013-2016). Due to the retrospective anonymized nature of this study, expressed written informed study consent from patients was not required.
Results
Demographics (Table 1)
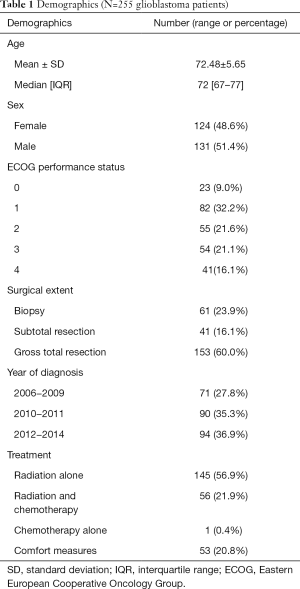
Full table
Two-hundred and fifty-five consecutive newly diagnosed GB patients aged 65 and older seen at a tertiary cancer centre from December 2006 to December 2014 were included. The median age was 72 years (range, 67–77 years). About 51% of patients were males and 49% were females. The percentage of patients with the following baseline ECOG performance status was as follows: 9%, 32%, 22%, 21%, and 16% for ECOG 0, 1, 2, 3, 4 respectively.
The majority of patients (60%) had a gross total resection based on the operative report and postoperative imaging. Twenty-four percent of patients underwent a biopsy and the remaining 16% had a subtotal resection.
In general, patients who had poor performance status and who were unlikely to derive significant survival or quality of life benefit with active therapy were managed with comfort measures (n=53). Other patients were treated with radiation alone (n=145), radiation and chemotherapy (n=56) or chemotherapy alone (n=1) with the intent to improve survival.
Survival
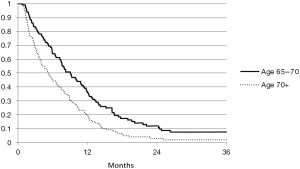
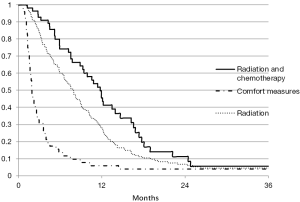
Overall median survival was 6 months (with an interquartile range of 1–19 months). Figure 1 shows survival by the following age intervals: 65–70 years, 70+ years. Figure 2 shows survival by treatment (comfort measures, radiation, radiation and chemotherapy, chemotherapy alone).
Hospitalizations
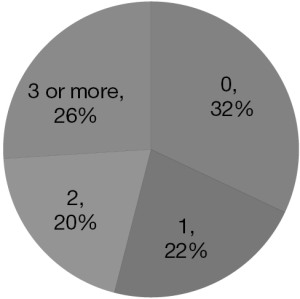
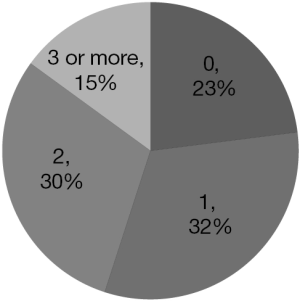
The majority of patients (68%) had at least one visit to the emergency department (Figure 3) and 77% had at least one admission to acute care (Figure 4), after diagnosis (taken as the date of first surgery showing GB).
Specific reasons for emergency department visits and admission to acute care were not adequately captured in the databases used.
The mean and median length of acute hospital stay per patient was 20.5 and 14 days respectively. The breakdown of duration of inpatient admission as a fraction of patient survival time is shown in Table 2.
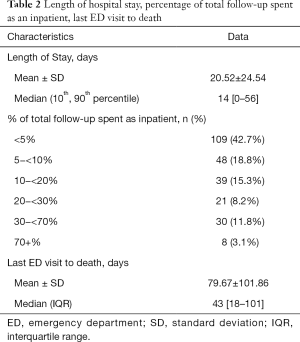
Full table
There was a mean of 79.7 days and a median of 43 days from the last emergency department visit to death (Table 2).
Palliative care
Based on physician services billing fee codes as palliative care, 60% of patients accessed physician services for palliative care.
Costs (all in Canadian dollars)
The mean and median hospitalization costs per patient was $87,625.79 and $47,673.00 respectively. The mean and median ambulatory costs per patient were $22,371.33 and $14,803 respectively. Mean and median OHIP billing costs per patient were $24,672.72 and $16,568.00 respectively. The mean and median costs for other health care per patient were $27,809.94 and $12,380, respectively. The mean and median total costs per patient were $162,479.78 and $125,511.00, respectively (Table 3).
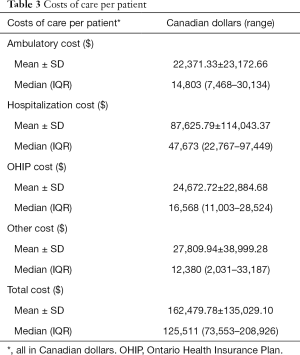
Full table
Risk factors for admission
Table 4 summarizes the risk factors for emergency department visits and hospital admission. Treatment with radiation or treatment with radiation and chemotherapy was associated with a relative risk of 2.31 (95% CI: 1.44–3.7; P=0.0005) and 2.19 (95% CI: 1.28–3.74; P=0.004), respectively for emergency department visits. Patients with baseline ECOG performance status of 0 or 1 had a RR of 2.73 (95% CI: 1.49–4.98; P=0.0011) and 1.75 (95% CI: 1.03–2.96; P=0.0372) for emergency department visits during the course of their illness as compared to patients with baseline ECOG performance status of 4. The majority of patients with ECOG performance status 4 were managed with comfort measures. Similarly, patients with a baseline ECOG 0 had a RR of 1.71 (95% CI: 1.06–2.77; P=0.0289) and patients with baseline ECOG 1 had a RR of 1.49 (0.98–2.26; P=0.0623) for hospital admission during the course of their illness as compared to patients with ECOG 4.

Full table
Discussion
Hospitalizations in GB patients
Although there have been publications on hospitalization in GB patients, ours is the only study which has examined hospitalizations and costs in GB patients 65 years and older from the time of diagnosis to death.
Rahman et al. (14) reported on 5,029 patients age 65 and older diagnosed with GB between 1999–2007 using SEER/Medicare-linked data in the United States. The authors reported that 21% were hospitalized at least 30 cumulative days between diagnosis and death. However, the study did not quantify the number of admissions or costs.
Rahman et al. (5) reported on 196 consecutive newly diagnosed GB patients age 23–90 years who underwent chemoradiation. Hospitalization outcomes were reported only during the period of chemoradiation and costs were not explored. The authors reported that 43% of patients were hospitalized during the chemoradiation period. Hospitalizations during the chemoradiation period were associated with shorter survival (adjusted hazard ratio 1.47; 95% CI: 1.01–2.13, P=0.043).
Diamond et al. (15) reported on GB patients, at least 18 years of age admitted within 1 month of death. They found that out of 385 GB patients, 42.6% were admitted within a month of death. Of these, 34% had ICU care.
In the present study, the proportion of patients who were hospitalized for a large fraction of their remaining survival time was low, as compared to prior reports in the literature. It may be hypothesized that strong palliative care supports in the community may have contributed to lowering this value as compared to published reports from other groups. However, despite the fact that GB is a uniformly terminal diagnosis, only 60% of our patients had a palliative care-associated billing code associated with their care. Opportunity exists to improve access to home palliative care supports and to evaluate whether this strategy lowers emergency room visits, acute care hospitalizations, quality of life and health care costs.
We found that the risk of emergency department visits and hospitalizations was associated with good baseline performance status and active treatment. This suggests that the morbidity of progressive disease and treatment puts these patients at risk of emergency room visits and hospitalizations, as compared to patients with shorter survival who are managed with comfort measures only.
These data underscore the magnitude of emergency department visits, hospitalization risk and health care costs associated with GB patients age 65 and older, particularly those who are selected for active therapy with the intent to improve survival.
Hospitalizations and goals of care
Challenges in the hospitalization of advanced cancer patients have also been highlighted by Bostanci et al. (16). The authors focused on the medical records of 39 advanced cancer patients who died in an acute care hospital. All the included patients had well established and predictably worsening disease and significant symptoms. In almost every case, admission to hospital followed advice from a doctor. Broader goals of care for these terminally ill patients were rarely documented at the time of hospital admission. The authors also noted that hospital discharge planning was frequently associated with family and inter-professional conflict. For some, it was difficult to transition from active investigations and treatments to end-of-life care. For others, although patients expressed a wish to have palliative care at home and to die at home, barriers included lack of home care supports and late recognition of dying with discharge planning delayed due to on-going investigations and considerations for further active therapies.
Wright et al. (17) examined a multi-site prospective cohort of 332 advanced cancer patients and their caregivers in the United States. Patients were followed from the time of enrollment to death. End of life discussions were associated with less aggressive medical care near death and earlier hospice referrals. Aggressive care was associated with worse patient quality of life and worse caregiver bereavement. Cancer patients who died in hospital or in the intensive care unit (ICU) were found to have worse quality of life compared to those who died at home. Bereaved caregivers for these cancer patients who died in hospital or in ICU were also found to be at increased risk of developing psychiatric illness (18).
Advanced care planning (ACP)
ACP allows patients to assign a substitute or surrogate decision maker to make clinical decisions on their behalf if they are not capable of making these decisions themselves (19). Due to the potentially rapid neurologic and cognitive decline of patients with GB, early discussions of ACP can have impact on treatment and care decisions (20). Incorporating ACP into the care of GB patients allows the patient, their family and their health care team to understand their unique concerns and preferences. ACP discussions can provide the patient and family with a better understanding of their illness and prognosis (19). This knowledge may lead to earlier Palliative Care referrals as well as less need for emergency room visits and acute care stays. ACP may ultimately improve both GB patients’ symptoms and their overall quality of life (20).
Home palliative care
A pilot project of palliative home care for primary brain tumour patients was reported in Italy (21). From 2000–2009, 572 patients were followed by a team of home care staff consisting of 1 neurologist, 2 physiotherapists, 2 psychologists, one social worker and 4 specialty nurses. Seventy percent of patients were managed at home until death. The hospitalization rate was lower (16.7%) in those who received the home care described above versus 38% of patients who did not. Costs of hospitalizations were also significantly lower in the group who received the specialized home care 517 € (95% CI: 512–522€) versus 24,076 € (95% CI: 24,040–24,112€) in those who did not.
For the subset of 197 patients with GB who received the specialized home care, 53.1% died at home, 34.4% died in hospice and 12.5% died in hospital. In 97% of cases, caregivers reported satisfaction with the home assistance. After 3 months, the Barthel Index (a measure of activities of daily living) improved in 43% of patients and 72% had an improvement in their quality of life scores in at least one item compared to baseline scores (22).
Limitations
The limitations of the present study are that the decision to hospitalize a patient is based on clinical judgment and that may vary among institutions and across communities based on palliative home care supports. Although we had detailed patient information such as performance status, extent of resection and treatment details in the patient database, we relied on a provincial database for hospitalizations and costs. As such, only hospitalizations and costs captured within the province were reported. Furthermore, the provincial database was inadequate with respect to reasons for emergency room visits and hospitalizations. The most common International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD10) codes was C719 (Brain, unspecified) for emergency department visits and C71 (malignant neoplasm brain) for hospital admissions.
The cost analysis only accounts for direct medical costs incurred by the single-payer universal provincial government health care system. Indirect costs such as income forgone because of impairment, disability or illness for the patient and caregiver(s) and other costs incurred by patients (such as copayment for medications, hospitalizations, canes/walkers/wheelchairs, transportation) are not addressed.
Future directions
In January 2014, a provincial palliative care integration project was initiated at our tertiary cancer centre. Hospital staff involved in the care of GB patients, were educated in a classroom setting with respect to approaches to palliative care. All GB patients were identified and since 2014, advanced care planning discussions are now initiated within the first few months of treatment. The process also involves integrating home care referrals earlier.
Whether strategies such as earlier palliative care discussions, improved palliative home care services and planning for direct palliative care unit or hospice placement when clinically relevant, will decrease emergency room visits, hospitalizations and quality of life requires further investigation.
Future directions include analyzing whether hospitalizations and costs have changed with the introduction of this provincial palliative care integration initiative at our cancer centre. As well, hospitalization rates and costs among different cancer centres can be compared using the existing administrative databases.
In addition, the development/use of a pretreatment Comprehensive Geriatric Assessment (CGA) tool may help to predict prognosis and toxicity (23). This tool is a multidimensional assessment that determines medical, functional, and psychosocial aspects of elderly patients. It is anticipated that the CGA will add to our present use of age and performance status to help guide GB management, especially in elderly patients. Furthermore, the CGA may help identify patients who have a higher risk of complications from treatment, emergency room visits and hospital admissions.
Conclusions
A large proportion of patients with GB over the age of 65 years will present to the emergency department and will have at least one admission to acute care during their illness. Most patients spent their post-diagnosis survival time as outpatients. However, the cost of inpatient care still contributes to approximately half of total costs of care for elderly GB patients. Whether strategies such as earlier palliative care discussions, improved palliative home care services and planning for direct palliative care hospital or hospice placement will decrease emergency room visits and hospitalizations requires further investigation.
Acknowledgements
This study was funded by the Sunnybrook Hospital Practice-Based Research Fund. The present study was supported by data supplied by the Institute for Clinical Evaluative Sciences (ICES) and Cancer Care Ontario (CCO).
Footnote
Conflicts of Interest: Dr. Arjun Sahgal has received honorarium for past educational seminars from Medtronic, Elekta AB, Accuray Inc., and Varian Medical Systems and research grants from Elekta AB. Dr. Sahgal also belongs to the Elekta MR Linac Research Consortium. The other authors have no conflicts of interest to declare.
Disclaimer: The results, opinions and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES, CCO or the Government of Ontario is intended or should be inferred.
References
- Arvold ND, Reardon DA. Treatment options and outcomes for glioblastoma in the elderly patient. Clin Interv Aging 2014;9:357-67. [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Tsang DS, Khan L, Perry JR, et al. Survival outcomes in elderly patients with glioblastoma. Clin Oncol (R Coll Radiol) 2015;27:176-83. [Crossref] [PubMed]
- Pan IW, Ferguson SD, Lam S. Patient and treatment factors associated with survival among adult glioblastoma patients: A USA population-based study from 2000-2010. J Clin Neurosci 2015;22:1575-81. [Crossref] [PubMed]
- Rahman R, Catalano PJ, Reardon DA, et al. Incidence, risk factors, and reasons for hospitalization among glioblastoma patients receiving chemoradiation. J Neurooncol 2015;124:137-46. [Crossref] [PubMed]
- Paszat L, Laperriere N, Groome P, et al. A population-based study of glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2001;51:100-7. [Crossref] [PubMed]
- Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol 2012;13:707-15. [Crossref] [PubMed]
- Perry JR, Laperriere N, O’Callaghan CJ, et al. A phase III randomized controlled trial of short-course radiotherapy with or without concomitant and adjuvant temozolomide in elderly patients with glioblastoma (CCTG CE.6, EORTC 26062-22061, TROG 08.02, NCT00482677). J Clin Oncol 2016;34:abstr LBA2.
- Weller M, Stupp R, Hegi ME, et al. Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro Oncol 2012;14 Suppl 4:iv100-8. [Crossref] [PubMed]
- Li J, Wang M, Won M, et al. Validation and simplification of the Radiation Therapy Oncology (RTOG) Recursive Partitioning Analysis (RPA) classification for glioblastoma (GBM). Int J Radiat Oncol Biol Phys 2011;81:623-30. [Crossref] [PubMed]
- Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med 2007;356:1527-35. [Crossref] [PubMed]
- Malmström A, Grønberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol 2012;13:916-26. [Crossref] [PubMed]
- Wodchis WP, Bushmeneva K, Nikitovic M, et al. Guidelines on person-level costing using administrative databases in Ontario. Working Paper Series 2013:1.
- Rahman R, Catalano PJ, Reardon DA, et al. Incidence, risk factors, and reasons for hospitalization among glioblastoma patients receiving chemoradiation. J Neurooncol 2015;124:137-46. [Crossref] [PubMed]
- Diamond EL, Panageas KS, Dallara A, et al. Frequency and predictors of acute hospitalization before death in patients with glioblastoma. J Pain Symptom Manage 2017;53:257-64. [Crossref] [PubMed]
- Bostanci A, Horey D, Jackson K, et al. Insights into hospitalisation of advanced cancer patients: A study of medical records. Eur J Cancer Care (Engl) 2016;25:190-201. [Crossref] [PubMed]
- Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions,patient mental health,medical care near death,and caregiver bereavement adjustment. JAMA 2008;300:1665-73. [Crossref] [PubMed]
- Wright AA, Keating NL, Balboni TA, et al. Place of death: Correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol 2010;28:4457-64. [Crossref] [PubMed]
- Song K, Amatya B, Voutier C, et al. Advance care planning in patients with brain tumours: A prospective cohort study. J Cancer Res Ther 2015;3:85-91. [Crossref]
- Fritz L, Dirven L, Reijneveld JC, et al. Advance Care Planning in Glioblastoma Patients. Cancers (Basel) 2016.8. [PubMed]
- Pace A, Di Lorenzo C, Capon A, et al. Quality of Care and Rehospitalization Rate in the Last Stage of Disease in Brain Tumor Patients Assisted at Home: A Cost Effectiveness Study. J Palliat Med 2012;15:225-7. [Crossref] [PubMed]
- Pompili A, Telera S, Villani V, et al. Home palliative care and end of life issues in glioblastoma multiforme: results and comments from a homogeneous cohort of patients. Neurosurg Focus 2014;37:E5. [Crossref] [PubMed]
- Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for geriatric oncology study. J Clin Oncol 2002;20:494-502. [Crossref] [PubMed]