A selective review of medical cannabis in cancer pain management
Introduction
Cancer patients often present with chronic pain, which may stem from direct tumour involvement, or present as a side effect of cancer treatment (1). As pain negatively impacts the physical, functional, and emotional domains of life, effective pain management strategies are essential for restoring and maintaining quality of life of cancer patients (2). Unfortunately, the current standard treatment regimens for chronic or neuropathic pain in end-stage cancer patients rely heavily on opioid analgesics, which are problematic for some patients (3,4). This can be due to a combination of factors, including differences in individual responses to these drugs, and the presence of serious side effects such as severe constipation, that may prevent the administration of sufficient doses for pain relief (3). In addition, imprudent dosing runs the dangerous risk of patients developing dependency, or overdosing on opioids (4). Therefore, identifying alternative classes of analgesics that can effectively manage pain in cancer patients is of great importance.
Alternative pharmacological interventions include prescription medications such as acetaminophen, or nonsteroidal anti-inflammatory drugs like ibuprofen (5). Non-medicated approaches include therapies such as acupuncture, physical therapy, in addition to psychological or behavioural approaches (6). In addition to the management strategies listed above, compounds derived from the plant species Cannabis Sativa L. have demonstrated the potential to alleviate pain. The most commonly studied examples include tetrahydrocannabinol (THC), and cannabidiol (CBD) from the family of compounds known as cannabinoids (7). These compounds are commonly administered via inhalation, orally as oils or oil-filled capsules, or oromucosally via sprays containing either THC alone, or a combination of THC:CBD (8). Several pre-clinical studies have been conducted in animal models, investigating the mechanism of cannabinoid modulation of pain pathways (9,10). One of the identified mechanisms is the interaction of these compounds with one of the body’s endogenous signalling systems, known as the “endocannabinoid” system (11). This system acts independently of the opioid pathway to control pain signalling, immune activation, and inflammation (11). While there is an abundance of existing anecdotal evidence of the analgesic properties of medical cannabis, its efficacy has not yet been validated through high-quality clinical studies that provide strong evidence supporting its utility in the clinical setting (12).
This selective review is an overview of clinical studies conducted historically and up until the present day that aimed to investigate the efficacy of medical cannabis in managing pain in advanced cancer patients.
Methods
A search of literature published on Medline between 1975 and 2017 through using key words including “cannabis”, “THC”, “CBD”, “Nabiximol”, “cancer”, and “pain” was conducted. Five clinical studies that evaluated the effect of THC or CBD on controlling cancer pain were evaluated for a selective review. Information regarding the study population, interventions, pain response, and side effects was reviewed and summarised.
Results
Patient populations and selection criteria
Five studies were selected based on their evaluation of cannabinoids to manage chronic pain in advanced cancer patients. An early pilot study conducted in 1975 by Noyes et al. assessed pain in ten advanced cancer patients (eight women and two men, average 51 years old) (13). In a similar pain management study, Noyes et al. compared the effects of THC and codeine in 36 cancer patients (consisting of 26 women and 10 men) (14). Non-study medications were withheld from patients from both studies by Noyes et al. during the study period (13,14). Johnson et al. conducted a multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of nabiximols and THC in patients with intractable cancer-related pain, using a well-distributed population of 177 advanced cancer patients, who recorded non-study breakthrough analgesics (15). In this study, the mean age, gender, primary disease sites, and pain classification were distributed similarly between the three treatment arms; THC, nabiximols, and placebo (15). In 2012, Portenoy et al. conducted a randomized, placebo-controlled, graded-dose trial involving 360 patients with advanced cancer, looking at the efficacy of THC or nabiximols. Patients were chosen based on having previously responded poorly to opioid analgesics, but were allowed to take breakthrough opioid analgesics as required (16). Patients who had received long-term methadone treatment for pain were excluded. Pain characteristics were categorized as mixed (48%), bone (24%), visceral (15%), and neuropathic (11%), and were distributed approximately equally across the study arms. Finally, Lynch et al. conducted a double-blind, placebo-controlled, crossover pilot trial including 16 cancer patients who had persistent neuropathic pain 3 months after their cancer treatment (17). These patients had an average 7-day pain intensity ≥4 on 0–10 NRS, stable concurrent analgesic treatment for 14 days prior to study initiation, and were not taking breakthrough analgesics.
Evaluation of pain
In the clinical studies of cannabinoids for cancer pain management included in this review, several methods of measuring changes in pain intensity were employed. Early studies by Noyes et al. used a 4-point pain scoring system in which 0= absent, 1= mild, 2= moderate, and 3= severe (13,14). Since then, many studies have employed the numerical rating scale (NRS) to evaluate pain on a 0–10 scale, with “0” representing “no pain” to “10” representing “pain as bad as you can imagine”. Patients with neuropathic pain studied by Lynch et al. completed the NRS at baseline, and the last day of each week of dosing (17). The change in NRS score from baseline to the week in which a stable dose was reached was used as the primary endpoint in determining cannabis efficacy. In the study by Johnson et al., patients used the NRS in addition to recording their long-term and break-through pain medications in a pain diary (15). Portenoy et al. asked patients to report their average pain on the brief pain inventory (BPI), as well as through an interactive voice recording system (16). The two remaining studies used the BPI to assess change in pain as the primary endpoint (18,19).
Efficacy of interventions
Overall, four out of the five studies found that cannabis was significantly associated with a decrease in cancer-associated pain. Table 1 presents a summary of the efficacy of THC or CBD on cancer pain.
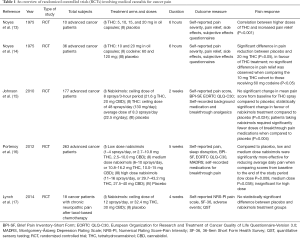
Full table
THC oil capsules and THC, CBD oromucosal sprays
Studies included in this review assessed the efficacy of THC oil capsules, and oromucosal sprays containing THC extract, or THC:CBD extract, also known as nabiximols. Since nabiximols have CBD in addition to THC, they may potentially target more pain pathways when compared to THC extract alone.
Two early clinical studies on the efficacy of THC extract in sesame oil capsules were published by Noyes et al. in 1975 (13,14). The first was a pilot study that identified a correlation between higher doses of THC and increased pain relief (P<0.001) (13). The second study found a significant difference in pain reduction between placebo and 20 mg THC (P<0.05), in favour of THC treatment (14).
Oromucosal sprays have been a common method of administration for cannabinoid-based medicines in clinical investigations, to date (12). Both THC extracts and nabiximols, administered oromucosally, were studied by Johnson et al. (15). They did not observe a significant change in mean pain score from baseline for THC spray compared to placebo, but did report a statistically significant change in favour of nabiximols treatment compared to placebo (P=0.024). In addition, they reported that patients taking nabiximols required significantly fewer doses of breakthrough pain medications when compared to placebo (P=0.004). Portenoy et al. found that compared to placebo, nabiximols were significantly more effective for reducing average daily pain when comparing scores from baseline to the end of the study period (P=0.038) (16). These findings are in contrast with the study by Lynch et al. in which there was no statistically significant difference between placebo and nabiximols treatment groups amongst the 16 patients experiencing cancer-related neuropathic pain (17).
Dosage
Studies assessed the efficacy of different doses of medication, or allowed patients to self-titrate up to a maximum dose, as dictated by study protocols.
Evaluation of the effect of 5, 10, 15, and 20 mg of THC in oil capsules by Noyes et al. found that the amount of pain relief increased with dose (13). Out of 10 patients in each cohort, 5 received substantial relief from 15 mg, and 7 patients received substantial relief from 20 mg. In the second study by Noyes et al., two different THC doses of 10 and 20 mg were compared to placebo and 60 mg codeine (14). A 60 mg dose of codeine is a standard daily opioid analgesic regimen used in the management of many pain types, including cancer pain (20). A significant difference in pain reduction was observed with the administration of 20 mg THC when compared to placebo (P<0.05). Additionally, no significant difference in pain relief was observed when comparing the 10 mg THC cohort to those receiving 60 mg codeine (P<0.05). This suggests the non-inferiority of 10 mg of THC in comparison to a commonly used opioid treatment.
Evaluation of the efficacy of THC oromucosal spray by Johnson et al. followed a self-titration method of dosing (15). Patients who used THC sprays used an average of 8.3 sprays/day, corresponding to 22.5 mg of THC/day following dose titration up to a ceiling dose of 48 sprays/day. Patients were considered to have reached their optimal dose upon experiencing relief of pain, or the development of side-effects. The authors found the optimal dose of THC reached across patients provided greater pain relief compared with placebo as measured by the average NRS pain score reduction (THC −1.01 vs. placebo −0.69) however, statistical significance was not reached (P=0.245).
In the three studies on nabiximols included in this review, self-titration was recommended up to a maximum dose of 8 sprays/3-hour period (15), and 11–16 sprays/day (16,17). In one of the studies, patients were divided into three dose groups categorized by titration ranges of mild (1–4 sprays/day, or 2.7–10.8 mg THC, 2.5–10.0 mg CBD), moderate (6–10 sprays/day, or 10.8–16.2 mg THC, 10.0–15 mg CBD), and high (11–16 sprays/day, or 29.7–43.2 mg THC, 27.5–40 mg CBD) (16). The doses found to produce significant pain relief include an average of 8.75 sprays/day (15), 1–4 sprays/day (16), and 6–10 sprays/day (16). It was observed that the high dose group of patients who utilised 11–16 sprays/day did not experience significant pain relief compared to placebo. Similarly, Lynch et al. found that at a high dose of an average of 8 sprays/day there was no significant pain relief observed in comparison to placebo (17).
Side effects and adverse events
Side effects reported in studies included in this review were consistent with those reported in literature investigating the use of cannabinoid-based therapies for several other indications (7). Table 2 summarises the five most commonly reported side effects of the five studies. In both studies by Noyes et al., side effects of 15 and 20 mg of THC included mental clouding (60–70%), drowsiness (70–100%), and euphoria (40–50%) (13,14). Not all side effects were experienced by all patients, and side effects tended to become more prevalent with higher doses.
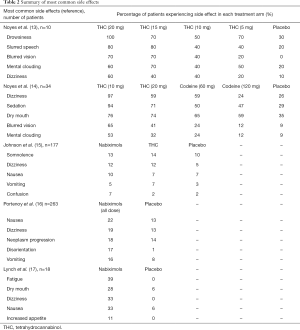
Full table
Common treatment-related adverse events reported by Johnson et al. include somnolence (nabiximols 13%, THC 14%, placebo 10%), dizziness (nabiximols 12%, THC 12%, placebo 5%), confusion (nabiximols 7%, THC 2%, placebo 2%), nausea (nabiximols 10%, THC 7%, placebo 7%), and hypotension (nabiximols 5%, THC 0%, placebo 0%) (15). These were reportedly more frequent in patients receiving the nabiximols extract and the THC only extract, when compared with placebo. The adverse events identified by Portenoy et al. were significantly more frequent in the higher nabiximols dose group, whereas little difference was observed between the low dose and placebo groups (17). Lynch et al. identified fatigue (nabiximols n=7, placebo n=0), dry mouth (nabiximols n=5, placebo n=1), dizziness (nabiximols n=6, placebo n=0), and nausea (nabiximols n=6, placebo n=1) to be the most common side effects, which were more often observed in the treatment arm compared to placebo, although the significance of this difference was not assessed. However, patients also reported that the majority of side effects were transient and mild, and could be reduced through adjusting treatment dose. Side effects did not lead to any study drop-outs (13-17).
Discussion
The paucity of clinical data available on medical cannabis for treatment of cancer pain is partly due its classification as a schedule I agent by the Controlled Substances Act in 1970, which restricted its investigation as a potential medical product (8). However, the few studies that were produced on the use of medical cannabis for cancer pain management have results that suggest it does possess therapeutic potential, and is at least worthy of further investigation.
There is a lack of dosing guidelines for the use of cannabinoid-based therapies in clinical practice. The ideal dosage would be one that provides effective pain management, but does not produce intolerable side effects. However, there are challenges in establishing this optimal dose in the advanced cancer patient population. One of these is inter-patient variability, in keeping with results from studies on narcotics and other prescription analgesics. As optimal doses were found to vary from patient to patient, physicians need to understand how to determine the correct dosage when prescribing to a new patient. In addition, advanced cancer patients are likely to present with complex symptomologies that make it difficult to accurately assess side effects derived from cannabis treatments, and are often taking multiple concurrent medications. That said, a number of these studies reported that observed side-effects tended not to be treatment-limiting, and could be controlled through dose titration, with pain relief in as little administration of 2.7–10.8 mg THC in combination with 2.5–10.0 mg CBD (17). This highlights the importance of establishing and validating a titration protocol that will allow researchers to identify effective and tolerated dosages in a safe and controlled manner.
Several studies presented in this review were underpowered due to small sample sizes, with three out of the five studies reviewed enrolling less than 50 patients. Therefore, the generalizability of the results may be limited, and future studies on medical cannabis are warranted to establish its efficacy and side effect profile in the cancer pain population. This includes additional efforts to identify the efficacies of specific cannabis compounds and their combinations, as well as ideal methods of administration through the assessment of relevant endpoints. Subsequent clinical trials should also consider the differences in cannabinoid pharmacokinetics and pharmacodynamics among individuals. Moreover, standardized and validated evaluation and reporting of cannabis-associated side effects is warranted in order to enable more accurate comparisons across studies. Ultimately, this will contribute to the development of clinical guidelines for the dosing and administration of cannabis as a pain medication for the large population of cancer patients in need of pain management, particularly those for whom alternative analgesics are insufficient, intolerable, or unsafe.
Conclusions
Current research shows that there is a potential role for medical cannabis in cancer pain management. However, the scale and quality of studies conducted to date are somewhat limited (12). Therefore, further research is needed to establish the efficacy of medical cannabis, either as an alternative to opiates or as an adjunctive therapy, and to identify the most appropriate methods of administration to achieve optimal therapeutic efficacy with minimal side effects.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund. This study was conducted in collaboration with MedReleaf.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kumar SP. Cancer Pain: A Critical Review of Mechanism-based Classification and Physical Therapy Management in Palliative Care. Indian J Palliat Care 2011;17:116-26. [Crossref] [PubMed]
- Katz N. The impact of pain management on quality of life. J Pain Symptom Manage 2002;24:S38-47. [Crossref] [PubMed]
- Trang T, Al-Hasani R, Salvemini D, et al. Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics. J Neurosci 2015;35:13879-88. [Crossref] [PubMed]
- Nersesyan H, Slavin KV. Current aproach to cancer pain management: Availability and implications of different treatment options. Ther Clin Risk Manag 2007;3:381-400. [PubMed]
- Schug SA, Chandrasena C. Pain management of the cancer patient. Expert Opin Pharmacother 2015;16:5-15. [Crossref] [PubMed]
- Syrjala KL, Jensen MP, Mendoza ME, et al. Psychological and behavioral approaches to cancer pain management. J Clin Oncol 2014;32:1703-11. [Crossref] [PubMed]
- Grotenhermen F, Müller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int 2012;109:495-501. [PubMed]
- Borgelt LM, Franson KL, Nussbaum AM, et al. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 2013;33:195-209. [Crossref] [PubMed]
- Ward SJ, McAllister SD, Kawamura R, et al. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol 2014;171:636-45. [Crossref] [PubMed]
- Deng L, Guindon J, Cornett BL, et al. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry 2015;77:475-87. [Crossref] [PubMed]
- Huang WJ, Chen WW, Zhang X. Endocannabinoid system: Role in depression, reward and pain control Mol Med Rep 2016;14:2899-903. (Review). [Crossref] [PubMed]
- Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015;313:2456-73. [Crossref] [PubMed]
- Noyes R Jr, Brunk SF, Baram DA, et al. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol 1975;15:139-43. [Crossref] [PubMed]
- Noyes R Jr, Brunk SF, Avery DA, et al. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther 1975;18:84-9. [Crossref] [PubMed]
- Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010;39:167-79. [Crossref] [PubMed]
- Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain 2012;13:438-49. [Crossref] [PubMed]
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014;47:166-73. [Crossref] [PubMed]
- Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 1983;17:197-210. [Crossref] [PubMed]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129-38. [PubMed]
- Straube C, Derry S, Jackson KC, et al. Codeine, alone and with paracetamol (acetaminophen), for cancer pain. Cochrane Database Syst Rev 2014.CD006601. [PubMed]


