Survival analysis of malignant epidural spinal cord compression after palliative radiotherapy using Tokuhashi scoring system and the impact of systemic therapy
Introduction
Approximately 60–70% of patients with metastatic cancer have spinal metastasis (1). Among the patients with spinal metastasis, approximately 2.5–10.0% of patients ultimately develop malignant epidural spinal cord compression (MSCC) during the course of their illness (1-4). Approximately 60–80% developed in the thoracic vertebrae, followed by the lumbosacral region and the cervical region (2). The median overall survival after MSCC diagnosis is around 4 to 6 months (2,3,5,6). The clinical consequences of MSCC are usually very drastic, including pain and neurological functional impairment which may not be reversible despite prompt treatment.
Local management options for MSCC generally include palliative radiotherapy, or surgical decompression and reconstruction for highly select cases. The optimal treatment mainly depends on prognosis prediction, neurological state and recovery potential. Palliative radiotherapy alone is usually adapted for those with limited neurological recovery potential and poor overall estimated survival, while aggressive surgical treatment and post-operative radiotherapy is advocated for those with more favorable prognosis, or who are expected to have higher neurological recovery potential (7). Advanced cancer patients with MSCC are often non-surgical candidates because of disease burden, cachexic state, altered mental conditions, co-morbidities, surgical risks, limited life expectancy and worries and concerns from care-givers.
Rades and colleagues studied the prognostic factors contributing to adverse outcomes of MSCC and established a scoring system for prognosis prediction after radiotherapy treatment. Short-course radiotherapy is suggested for MSCC patients with the poorest prognosis. However, the scoring system carries limitations for routine clinical practice (4,8). Mazaranno and colleagues have examined the outcome of short-course and long-course palliative radiotherapy for poor prognosis MSCC patients (5,9). The studies showed that despite different schedules, there was little difference in various outcomes of MSCC, including neurological function, pain control and ambulatory status. While single-Fr palliative radiotherapy may be preferred over multi-Fr in daily practice for poor prognosis MSCC patients, this group of patients has not yet been clearly identified. Single-Fr radiotherapy not only helps to reduce the potential side effects and unnecessary worries caregivers may have about radiotherapy, it also helps to alleviate the burden on the whole radiotherapy service.
The modified Tokuhashi scoring system (10) and the Tomita scoring system (11) are internationally validated pre-operative scoring systems to predict survival of MSCC patients, they were developed to maximize the chance of neurological recovery and ambulatory status, and to avoid unnecessary surgical procedures in patients with poor prognosis.
In this retrospective review, we examined if the Tokuhashi predictive system can be adapted for use in patients receiving palliative radiotherapy for MSCC by stratifying the patients into two different prognostic groups, both of whom were considered non-surgical candidates. The primary objective was survival analysis against various prognostic factors. The results of the study may provide additional clinical information to aid clinicians to decide on a radiotherapy schedule for advanced MSCC patients.
Methods
From January 2014 until May 2015, all MSCC patients who received radiotherapy were documented. Eligible patients were identified after excluding those who were treated surgically. Haematological malignancies were also excluded as they are chemosensitive and not accounted for by the Tokuhashi scoring system. All patients received urgent palliative radiotherapy when the service was available. Patient medical records were reviewed and studied. Each individual’s best Tokuhashi score was estimated, and patients were stratified into two groups (group 1: below 9, group 2: above 8) only, instead of the three groups according to the original scoring system. This is because separating patients with expected survival of 6–12 months or longer was deemed clinically insignificant in this setting. The predicted survival of patients scoring below 9 would be less than 6 months. Other potential prognostic factors included age, disease primary (primary breast or prostate cancer, or primary lung cancer, or others), non-visceral metastasis; baseline lower limb power [Medical Research Council (MRC) grading], prior radiotherapy; radiotherapy fractionation (multi-Fr or single), and subsequent systemic therapy, including chemotherapy, targeted therapy or hormonal therapy. The MRC muscle strength scale is widely used for MSCC, apart from grading systems used in other publications (5,9,12,13). In general, for patients with MSCC, individuals would be considered to have ambulatory difficulties if the lower limb power is only MRC grade 3 over 5 or lower.
Survival rates were calculated by the Kaplan-Meier method. Log-rank test was used to compare survival among different prognostic factors. Prognostic factors found to be significantly correlated with survival were subsequently analysed in multivariate analysis using a Cox proportional hazards model.
Results
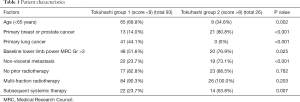
The data from 119 patients was eligible for analysis. A total of 116 patients had already succumbed (97.5%). The overall median survival was 55 days (range, 4–576 days). Patient characteristics are listed in Table 1.
Full table
Most of the patients (80.7%) had an MRI scan for MSCC diagnosis, the remaining patients (13.4%) had a PET or CT scan. The thoracic spine was most commonly affected, involving 87 patients (73.1%); the cauda-equina and cervical spine were involved in 33 (27.7%) and 23 patients (19.3%), respectively. Ninety-three patients (78.2%) belonged to Tokuhashi group 1. The median dose delivered was 25 Gy in 5 Frs [range, 7 Gy in 2 Frs to 40 Gy in 10 Frs (to the cauda equina)]. Only 9 patients (7.6%) received single-Fr radiotherapy, all belonging to group 1. One patient received 7 Gy in 2 Frs, which was radio-biologically similar to single 8 Gy. Fifty-one (42.9%) patients had a best lower limb power of grade 3 or lower before radiotherapy, which meant they were unlikely to be ambulatory at that time. Nineteen patients (16.0%) had prior irradiation to the site of MSCC, median time to re-irradiation was 262 days.
As compared to patients in group 2, patients belonging to group 1 were of younger age, had predominantly lung primary, worse baseline motor function, more visceral involvement, and much fewer of them received systemic therapy (Table 1).
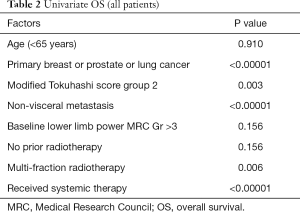
In univariate survival analysis, group 1 patients had poorer overall survival compared to group 2 (Table 2); median survival was 49 and 108 days, respectively (P=0.003) (Figure 1A). Other factors associated with better survival in univariate analysis also include: primary breast, prostate or lung cancer over other types of cancer (P<0.00001), multi-Fr radiotherapy (P=0.006), non-visceral metastasis (P<0.00001), no prior radiotherapy to same site (P=0.017) and subsequent systemic treatment (P<0.00001) (Figure 1B).
Full table
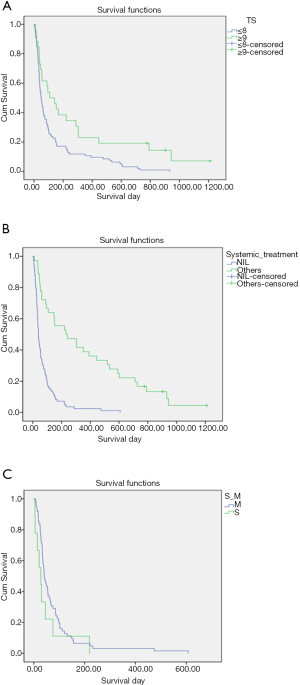
However, only subsequent systemic treatment [hazard ratio (HR) =0.407; 95% confidence interval (CI), 0.236–0.702; P=0.001], non-visceral metastasis (HR =0.608; 95% CI, 0.387–0.956; P=0.031) and primary lung or breast or prostate cancer (P=0.029) were associated with better survival in multivariate analysis (Table 3).

Full table
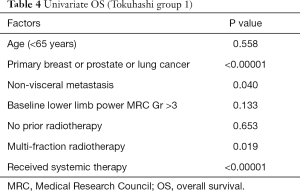
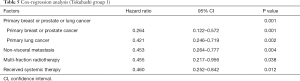
Further analysis was conducted among group 1 patients. In univariate analyses, primary diagnosis, multi-Fr radiotherapy, systemic treatment and non-visceral metastasis were associated with better survival (Table 4). In the Cox-regression model, primary breast or prostate cancer (HR =0.264; 95% CI, 0.122–0.572; P=0.001) or lung cancer (HR =0.421; 95% CI, 0.246–0.719; P=0.002), non-visceral metastasis (HR =0.453; 95% CI, 0.264–0.777; P=0.004), multi-Fr (HR =0.455; 95% CI, 0.217–0.956; P=0.038) and subsequent systemic therapy (HR =0.460; 95% CI, 0.252–0.842; P=0.012) all remained to be significantly correlated with overall survival (Table 5).
Full table
Full table
The survival of a subset of patients in group 1 without subsequent systemic therapy was dismal (median survival only 40 days) and not altered by radiation therapy schedule (P=0.189) (Figure 1C).
Discussion
The key to decide on the optimal treatment for MSCC patients largely depends on prognosis prediction. It is generally accepted that palliative radiotherapy alone is the treatment choice for patients with expected limited survival, probably in terms of a few months, so as to avoid unnecessary surgical procedures (14). Quoted median overall survival for MSCC patients treated by palliative radiotherapy is said to be between 3–6 months (4-6).
Our result is not powered enough for any effect of radiotherapy schedule on survival. Most of the clinical evidence so far confers no significant clinical association between overall survival and radiotherapy schedule in poor prognosis MSCC patients. Maranzano and colleagues have conducted two prospective randomised studies in poor prognosis MSCC patients: the first study compared 30 Gy in 8 Frs and 16 Gy in 2 Frs, and the second compared 16 Gy in 2 Frs and 8 Gy single-Fr (5,9). They showed that 8 Gy single-Fr was similar to multi-Fr in terms of pain, motor function, survival and toxicity. Another prospective study by Rades and colleagues investigated the outcomes of MSCC patients treated by either short-course (either 8 Gy single-Fr or 20 Gy in 5 Fr) or long-course (30 Gy in 10 Fr, 37.5 Gy in 15 Fr or 40 Gy in 20 Fr) radiotherapy and observed similar results, with the additional finding that local control could be improved after long-course treatment (13). Nevertheless, prior studies have shown that primary breast or prostate cancer, no visceral metastases, no other bone metastases, being ambulatory before radiotherapy, interval of at least 15 months between tumor diagnosis and MSCC, and less than 14 days of developing motor deficits before radiotherapy, are favorably associated with survival (4,8).
The majority (92.4%) of our patients received multi-Fr radiotherapy. In clinical practice, multi-Fr radiotherapy is usually favored over single-Fr for MSCC. This is attributed to a lack of clear clinical guidance to identity the group of poor-prognosis MSCC patients that clinicians feel reassured to offer single-Fr radiotherapy, as there is known to be an increased risk of in-field failure if the patients survive long enough after an initial course of radiotherapy (1,15). In Mazaranno’s studies (5,9), the poor prognosis patients were defined as having unfavorable histologies (e.g., lung, kidney, gastrointestinal, head and neck carcinoma, melanoma or sarcoma) or favourable histologies (e.g., lymphoma, seminoma, myeloma, breast or prostate carcinoma), provided that motor or sphincter dysfunction and/or low performance status was also manifest. In Rades and colleagues’ prospective study, no particular poor prognosis MSCC patient group was defined (13). Subsequently, Rades and colleagues established a prognostic scoring system for MSCC patients receiving palliative radiotherapy (8,16). It separates MSCC patients into three prognostic groups. The estimated survival at 6 months for group 1 (20–30 points), group 2 (31–35 points) and group 3 (36–45 points) was 16%, 48% and 81%, respectively. It was suggested that group 1 patients should receive short-course radiotherapy, while long-course treatment was recommended to group 3; the treatment to group 2 was left up to the clinicians’ decision. However, this scoring system may not be a popular tool for routine clinical practice. Firstly, one may find the scoring too complicated, as the total score is up to 45, with different scoring points among the six prognostic factors. Secondly, there is no clear treatment suggestion for patients belonging to group 2.
On the other hand, the Tokuhashi scoring system with a total score of 15, which accounts for factors associated with both the spine and the general disease status, is simpler and more convenient to use in routine clinical practice, despite the fact that it was developed for surgical treatment of MSCC rather than for radiotherapy (10). This pre-operative prognostic scoring system showed a survival of less than 6 months, 6 months or more and 1 year or more in 89.0% of patients scoring 0–8, 78.6% of patients scoring 9–11 and 87.5% of patients scoring 12–15, respectively. Among our whole patient sample, Tokuhashi scoring only correlated with better survival in univariate analysis, but not in multivariate survival analysis. Non-visceral metastasis and systemic treatment were correlated with better survival in multivariate analysis; systemic treatment was highly significant (P=0.001). All nine patients who received single-Fr belonged to group 1; they also did not receive subsequent systemic therapy. This was believed these patients were at the very advanced stage of disease with very short life expectancy.
Among group 1 patients, there were several factors associated with better survival in the multivariate analysis, including primary breast, prostate or lung cancer, multi-fractionation radiotherapy, subsequent systemic treatment and non-visceral metastasis. There were only 22 patients (23.7%) in this group who had received systemic therapy, but this factor was highly significant in the model (P=0.012). Forty-one patients (44.1%) in this group suffered from lung cancer, 14 (34.1%) of them received systemic treatment. Eight patients received tyrosine kinase inhibitors, four patients received sequential chemotherapy and tyrosine kinase inhibitors and the remaining four received systemic chemotherapy. The median survival of this group was up to 230 days. Thirteen patients (14.0%) in this group had breast or prostate cancer, 6 of them (46.2%) received systemic therapy; the median survival was 151 days. It was only marginally significant (P=0.038) for multi-Fr over single-Fr radiotherapy, although 84 patients (90.3%) received multi-fractionation radiotherapy.
To date, there is insufficient information concerning the effect of systemic therapy on survival of MSCC patients receiving palliative radiotherapy. Rades and colleagues (13) showed a trend towards improved local control, and significant higher survival rates at 6 and 12 months, with the use of bisphosphonates in univariate analysis. However, they explained that the findings could be attributed to the fact that bisphosphonates were generally administered to patients with a relatively favorable expected survival. Nevertheless, as there is no strong clinical evidence showing a survival benefit of bone modifying agents in treating metastatic solid tumors, we did not consider bone modifying agents, including bisphosphonates, as systemic therapy in this study.
In our study, the effect of systemic treatment was shown to be strongly correlated with overall survival in the whole patient sample, and also in the Tokuhashi group 1 patients. The median survival of the patients who had received systemic therapy after radiotherapy in group 1 was up to 151 days, compared with only 40 days in those receiving best supportive care. In the multivariate analysis of patients belonging to group 1, primary lung cancer was found to be associated with longer survival than other primary sites (with the exception of breast or prostate). The estimated median overall survival (75 days) of those with primary lung cancer was the same as primary breast or prostate cancer; and similar association was also found among the whole patient sample. This is believed to be due to the very significant positive impact of systemic treatment, despite MSCC, in these highly selected patients.
It’s known that metastatic breast and prostate cancer are usually associated with favorable prognosis, even in MSCC (8,10). These tumors are sensitive to treatments including chemotherapy, hormonal therapy and radiotherapy, and they score high in the published prognostic scoring systems. On the other hand, metastatic lung cancer is notoriously associated with unfavorable prognosis, especially in the MSCC setting (8,10). It gives the lowest score on the Tokuhashi and Rades prognostic systems.
Our findings on the impact of systemic therapy on survival may suggest incorporating this factor into the known MSCC prognosis prediction models. To date, it is not well studied and accounted for by any of the validated prognostic scoring systems (8,10,17). Recently, in a retrospective review of MSCC in lung cancer patients who received surgical decompression, the authors also found a positive correlation between adjuvant targeted therapy and survival in multivariate analysis (18).
Novel chemotherapeutic agents and targeted therapy have prolonged the median survival of metastatic non-small lung cancer (NSCLC) to beyond 1 year (19). In recent years, the use of tyrosine kinase inhibitors for NSCLC patients harboring certain epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations further improved the treatment response rates to over 60–70% (20,21), and the median progressive-free survival to over 1 year. Such similar advances in treatment have also occurred in a wide range of other solid tumors which are classically less responsive to systemic therapy, e.g., kidney cancer and melanoma. Moreover, very recent breakthroughs in immunotherapy have shown promising long-term disease control in melanoma (22,23), as well as other solid tumors, including lung and kidney cancer (24,25). As a result, the classical importance of primary diagnosis in the existing prognostic scoring systems will face serious challenge from the availability of effective systemic therapy. It is believed that for classical poor prognosis MSCC patients, with a performance status still suitable to receive effective systemic therapy, the likelihood of prolonged survival is higher; therefore, local control is of greater importance (1,13,15,16). In these cases, multi-Fr or long-course radiotherapy is preferred. On the other hand, for patients belonging to Tokuhashi group 1, and without reasonable systemic treatment options, their estimated overall survival was dismal, despite single- or multi-Fr radiotherapy (Figure 1C); in these cases, single-Fr treatment shall be preferably considered for them. The results and suggested management decisions from this study should be examined and validated by future studies and prospective data.
There are several limitations of this study. Firstly, the data and results were biased. In the whole sample, only nine patients received single-Fr radiotherapy, all were in group 1, and none of them received subsequent systemic therapy; one-third of them had prior radiotherapy to the MSCC sites. It is strongly believed that the decision of radiotherapy schedule was largely determined by the clinician’s prediction of the patient’s prognosis, depending on general prognostic factors and any plan of systemic treatment, rather than according to a particular prognostic scoring system. Secondly, a significant proportion of our patients were in the very advanced stage of their disease course; there were many other factors, including metastasis to other body sites, disease burden, cachexia, pain, medication use and physical de-conditioning affecting the patient’s ambulatory status, which is one of the known prognostic factors in MSCC. Thus, it was decided to use limb power function in terms of MRC grading instead. The data on duration of motor deficit before radiotherapy was not comprehensive in our patient data, so it is not evaluated in the survival analysis, though it has been shown to be another prognostic factor (4,8).
Conclusions
MSCC comprises a very heterogenous group of patients, even under the Tokuhashi grouping, systemic therapy or visceral metastasis may be more important prognostic factors. Further studies are necessary to better select the poor risk group. In clinical practice, single-Fr radiation therapy could be considered in Tokuhashi group 1 patients due to expected short survival, especially those without reasonable systemic treatment options.
Acknowledgements
Accepted for poster presentation at the 2016 Annual Meeting-American Society for Radiation Oncology (ASTRO), Boston Convention and Exhibition Center, Boston, Massachusetts, USA, 25th–28th September 2016.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the New Territories West Cluster Clinical & Research Ethics Committee (NTWC/CREC/17040). Only hospital numbers are recorded in this study, no other patient identifiable particulars are present. All the data is processed and stored in password protected computer or soft-wares. Only the authors and the clinical research assistant team of the institute have access to the data.
References
- Lam TC, Uno H, Krishnan M, et al. Adverse Outcomes After Palliative Radiation Therapy for Uncomplicated Spine Metastases: Role of Spinal Instability and Single-Fraction Radiation Therapy. Int J Radiat Oncol Biol Phys 2015;93:373-81. [Crossref] [PubMed]
- Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol 2005;6:15-24. [Crossref] [PubMed]
- Loblaw DA, Mitera G, Ford M, et al. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys 2012;84:312-7. [Crossref] [PubMed]
- Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol 2006;24:3388-93. [Crossref] [PubMed]
- Maranzano E, Bellavita R, Rossi R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol 2005;23:3358-65. [Crossref] [PubMed]
- Hoskin PJ, Grover A, Bhana R. Metastatic spinal cord compression: radiotherapy outcome and dose fractionation. Radiother Oncol 2003;68:175-80. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Rades D, Douglas S, Veninga T, et al. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer 2010;116:3670-3. [Crossref] [PubMed]
- Maranzano E, Trippa F, Casale M, et al. 8Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol 2009;93:174-9. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186-91. [Crossref] [PubMed]
- Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298-306. [Crossref] [PubMed]
- Medical Research Council. Aids to examination of the peripheral nervous system. Memorandum no. 45. London: Her Majesty’s Stationary Office; 1976.
- Rades D, Lange M, Veninga T, et al. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 2011;79:524-30. [Crossref] [PubMed]
- Choi D, Crockard A, Bunger C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J 2010;19:215-22. [Crossref] [PubMed]
- George R, Jeba J, Ramkumar G, et al. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev 2015.CD006716. [PubMed]
- Rades D, Veninga T, Bajrovic A, et al. A validated scoring system to identify long-term survivors after radiotherapy for metastatic spinal cord compression. Strahlenther Onkol 2013;189:462-6. [Crossref] [PubMed]
- van der Linden YM, Dijkstra SP, Vonk EJ, et al. Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer 2005;103:320-8. [Crossref] [PubMed]
- Tang Y, Qu J, Wu J, et al. Metastatic Spinal Cord Compression from Non-Small-Cell Lung Cancer Treated with Surgery and Adjuvant Therapies: A Retrospective Analysis of Outcomes and Prognostic Factors in 116 Patients. J Bone Joint Surg Am 2015;97:1418-25. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Greenhalgh J, Dwan K, Boland A, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev 2016.CD010383. [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Ugurel S, Röhmel J, Ascierto PA, et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies. Eur J Cancer 2016;53:125-34. [Crossref] [PubMed]
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]


