Gender differences in pain and patient reported outcomes: a secondary analysis of the NCIC CTG SC. 23 randomized trial
Introduction
Due to the differences in physiology and psychology, as well as economic and social circumstances, men and women may differ in their health risks and outcomes (1). This includes the experience of pain, where experimental studies have found gender differences in pain thresholds, tolerance, and responses to both pharmacological and non-pharmacological pain interventions (2-6). Contributing factors include biological differences, such the menstrual cycle in females and the influence from the complex interaction of sex-specific hormones that lead to differences in pain perception (4). On top of this, different social expectations of masculinity and femininity may also lead to gender-specific differences in the reporting of pain. Identification of the areas in which males and females differ is required in order to guide clinical practice to understand and better help patients.
Pain is one of the most common and distressing symptoms experienced by patients with advanced cancer (5). Unrelieved pain can disrupt and interfere with many activities of daily living, quality of life (QOL), and mood (6). In cancer patients with painful bone metastases, palliative radiotherapy is an effective treatment option in improving pain and QOL. However, there are few studies that assess potential differences in responses between males and females.
In the conclusion of the NCIC Clinical Trials Group (NCIC CTG) Symptom Control SC.23 study in cancer patients with painful bone metastases, treatment with dexamethasone significantly reduced pain flare incidence in comparison to placebo (7). The present study is a secondary analysis based on the SC. 23 trial, with the objectives of exploring differences in pain between men and women, including differences in response to palliative radiotherapy, and its effect on other patient reported QOL outcomes.
Methods
Patients were enrolled in the double-blind, placebo controlled study conducted across 23 Canadian cancer centres (7). Patients were eligible if they were at least 18 years of age, receiving a single 8 Gy dose of radiation for bone metastases at one or two locations, and had pain severity of at least 2 out of 10 in the Brief Pain Inventory (BPI) at the treatment site(s) (8,9). Ineligibility criteria included Karnofsky Performance Status (KPS) below 40, use of corticosteroids concurrently or within 7 days of study initiation, evidence of pathological or impending fracture, or spinal cord compression. Written consent was obtained for all patients enrolled and approvals from the research ethics boards of the 23 cancer centers involved in this study were obtained. The study was approved by provincial Ontario Cancer Research Ethics Board (OCREB) (No. 10-094).
Demographic information including patient gender, age, KPS, primary malignancy site, and treatment site was collected. Patients were randomly assigned to 1 of 2 arms. The treatment arm consisted of two tablets of 4 mg of dexamethasone or placebo taken at least 1 h before start of radiotherapy then every day for 4 days after radiotherapy. Patients kept a pain diary in which they recorded worst pain scores (WPS) on the BPI from a scale of 0 to 10, analgesic intake before treatment and daily for 10 days after radiotherapy. WPS was classified as mild [1–5], moderate [6] and severe [7–10] (10). Analgesic intake was converted into an oral morphine equivalent (OME) daily.
Response to radiotherapy was defined according to the International Bone Metastases Consensus Endpoint definitions (11). In brief, complete response (CR) refers to no pain at treatment site with no increase in analgesic intake; partial response (PR) refers to WPS reduction of at two or more without increase in analgesic intake, or no increase in WPS but reduction in 25% or more of analgesic intake when compared to baseline. Pain progression (PP) refers to at least a 2-point increase in the WPS without decrease in analgesic intake, or an increase of 25% or more of analgesic intake but no change in WPS. Remaining patients were characterised as having stable pain.
Within 7 days before treatment, and at 10 and 42 days after treatment, patients completed the European Organisation for Research and Treatment of Cancer (EORTC) QOL bone metastases module (QLQ-BM22) (12,13) and the EORTC QOL Core-15-Palliative (QLQ-C15-PAL) (14,15) questionnaires. The QLQ-BM22 is a questionnaire validated specifically for patients with bone metastases (12,13). It assesses two symptomatic (painful characteristics and painful sites) and two functional (functional interference and psychosocial aspects) scales. The QLQ-C15-PAL is the short form of the QLQ-C30 and was designed specifically in the palliative population (14,15). It contains single and multi-item scales that assess symptoms and functioning. All items in both scales, except for the global QOL item in the QLQ-C-15-PAL were scored on a Likert scale from 1 (not at all) to 4 (very much). Higher values indicate worse symptoms, but better functioning. All scores were linearly converted into a scale of 0–100 according to EORTC guidelines (16). A score change of 10 or more on this scale represented a clinically significant difference (17).
Descriptive statistics including mean and standard deviation were used to describe the patient baseline characteristics, scores, and score changes in the QLQ-BM22 and QLQ-C15-PAL at baseline and 42-day follow-up. Comparisons of patient demographics, performance status, analgesic consumption, BM22 and C15 were compared between gender groups with the 2-sample t-test for continuous variables and the Chi-squared test for categorical variables. Multiple linear regression models were used to check the difference in changes in QOL scores between gender groups adjusting for the baseline demographics and primary disease sites. P values <0.05 were considered statistically significant. All analyses were done with SAS, version 9.2.
Results
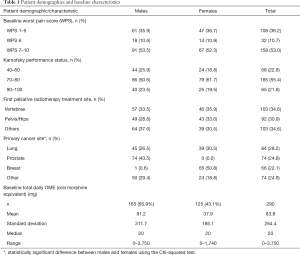
There were 298 patients (170 male, 128 female) with a median age of 69 years. Baseline demographics are presented in Table 1. The most common primary cancer sites were lung, prostate and breast. Most patients had a baseline WPS of 7–10, and KPS of 70 or 80. At baseline, there were no significant differences between males and females in WPS, KPS, or first palliative radiotherapy treatment site. As would be expected, male and female patients differed significantly in the common primary cancer sites of prostate and breast.
Full table
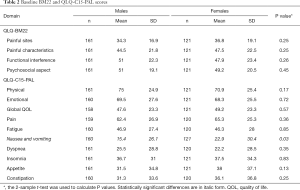
At baseline, there were no differences between males and females in the scores of the four QLQ-BM22 domains (Table 2). However, on the QLQ-C15-PAL, males and females differed significantly in the domain of nausea and vomiting, with females scoring higher than males (P=0.03).
Full table
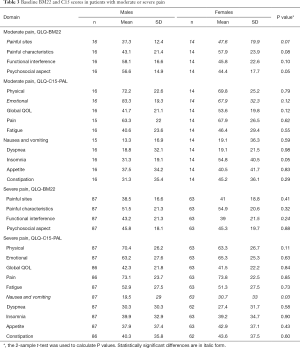
To investigate whether severity of baseline pain influenced gender-specific outcomes after radiotherapy, males and females were also compared across the QLQ-BM22 and QLQ-C15-PAL domains after being stratified by severity of their baseline WPS (Table 3). In patients with moderate pain, the only significant difference between males and females was in the domain of painful sites of the QLQ-BM22, where females reported worse score than males (P=0.01). In patients with severe pain, females reported worse nausea and vomiting scores (P=0.03).
Full table
There were no differences between males and females in response to radiotherapy when evaluated at day 42 (female: 41.2% vs. male: 35.9%, P=0.36, Table 4). Males and females had similar overall rates of CR, PR, and non-response. When stratified according to pain severity categories at baseline, no significant differences in radiotherapy response were identified.

Full table
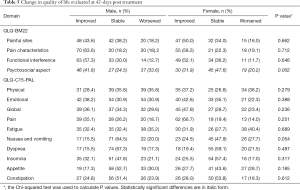
Males and females were evaluated for changes in QOL domains from baseline to day 42 after radiotherapy (Table 5). No differences existed between males and females except in the item of psychosocial aspect in the QLQ-BM22 questionnaire, in which a higher proportion of males reported either improvement or worsening of this domain (P=0.002).
Full table
Table 6 presents the analysis of the change in QOL among males and females separated according to radiotherapy responders and non-responders. Among the responders, higher proportions of males reported either improvement or deterioration in psychosocial aspect from the QLQ-BM22 (P=0.04), while more females reported improvement in emotional aspect of the QLQ-C15-PAL (P=0.03). Among the non-responders, there were significant differences in psychosocial aspect of the QLQ-BM22, again with higher proportion of males reporting either improvement or worsening in this domain when compared to females (P=0.04). On the other hand, higher proportion of females reported either improvement or worsening of the dyspnea item in the QLQ-C15-PAL (P=0.02).
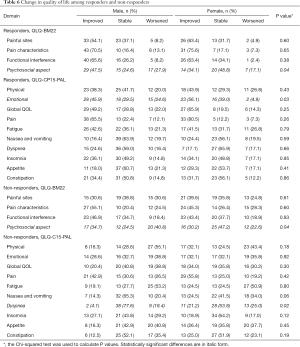
Full table
Discussion
Our study identified limited differences between men and women in baseline characteristics and QOL domains and pain levels, as well as in response to palliative radiotherapy in terms of pain reduction and QOL domains. This finding held after stratification of patients according to severity of symptom presentation at baseline.
Existing literature on gender differences in pain presentation and QOL in cancer patients have been inconclusive. The patient population in Wong et al. was similar to the present study (18). The authors investigated 396 patients with advanced cancer who underwent palliative radiotherapy for painful bone metastases. They found that female gender was associated with better baseline functional interference and painful characteristics domains on the QLQ-BM22. However, males and females did not significantly differ in QOL changes from baseline to follow-up. Our study yielded similar results in that there were no differences between males and females in all QLQ-BM22 domains except for psychosocial aspect. Since the baseline WPS and KPS were not significantly different between males and females, a plausible explanation may be that males were more responsive to changes in pain in terms of psychosocial aspects. However, unlike the results from Wong et al., we also did not identify any differences in patient-reported QOL domains at baseline.
A second study that utilized the QLQ-BM22, in addition to the QLQ-C30-PAL, was conducted by Püsküllüoğlu et al. (19). They found that in 110 Polish patients with cancer, men reported higher pain, worse fatigue, and worse nausea and vomiting compared to women. Moreover, at the 2-week follow-up, men also reported worse outcomes in the BM22 functional interference domain, and the QLQ-C30 social, emotional, and cognitive functioning domains. On the other hand, a study by Montague et al. of 96 cancer patients identified no differences between males or females in any of the QLQ-C30 subscales (20). Moreover, there were no gender differences in the duration and quality of breakthrough pain, or in the efficacy of pain medications.
While several studies on gender differences have been conducted using the QLQ-C30 questionnaire, this present study is the first to use the abbreviated QLQ-C15-PAL questionnaire. We identified that males and females report similar scores in the symptomatic and functional domains at baseline. In all but the psychosocial aspect domain, males and females report similar changes from baseline to follow-up. However, our finding that males report more improvement and deterioration in psychosocial aspect may be limited in its clinical implication, and will require further investigation to assess any potential significance. Overall, inconsistent findings in this area warrant further research to establish whether gender affects pain and QOL in cancer patients. This will help inform the interpretation of patient-reported symptoms and health outcomes, as well as guide improvements in health care practice that reflect the potentially different needs of each gender.
Conclusions
In cancer patients with bone metastases undergoing palliative radiotherapy, there appears no significant difference in general between genders in symptom presentations, patient reported outcomes and response to radiation. Therefore, men and women should be considered equally in consideration for palliative radiotherapy for painful bone metastases.
Acknowledgements
This study was supported by the NCIC CTG’s programmatic grant from the Canadian Cancer Society Research Institute. We thank all the patients who participated, and the research teams.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by provincial Ontario Cancer Research Ethics Board (OCREB) (No. 10-094). Written consent was obtained for all patients enrolled and approvals from the research ethics boards of the 23 cancer centers involved in this study were obtained.
References
- Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007;132 Suppl 1:S26-45. [Crossref] [PubMed]
- Alabas OA, Tashani OA, Tabasam G, et al. Gender role affects experimental pain responses: a systematic review with meta-analysis. Eur J Pain 2012;16:1211-23. [Crossref] [PubMed]
- Musey PI Jr, Linnstaedt SD, Platts-Mills TF, et al. Gender differences in acute and chronic pain in the emergency department: results of the 2014 Academic Emergency Medicine consensus conference pain section. Acad Emerg Med 2014;21:1421-30. [Crossref] [PubMed]
- Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111:52-8. [Crossref] [PubMed]
- Burton AW, Fanciullo GJ, Beasley RD, et al. Chronic pain in the cancer survivor: a new frontier. Pain Med 2007;8:189-98. [Crossref] [PubMed]
- Katz N. The impact of pain management on quality of life. J Pain Symptom Manage 2002;24:S38-47. [Crossref] [PubMed]
- Chow E, Meyer RM, Ding K, et al. Dexamethasone in the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases: a double-blind, randomised placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:1463-72. [Crossref] [PubMed]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129-38. [PubMed]
- Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 1983;17:197-210. [Crossref] [PubMed]
- McDonald R, Ding K, Chow E, et al. Classification of painful bone metastases as mild, moderate, or severe using both EORTC QLQ-C15-PAL and EORTC QLQ-BM22. Support Care Cancer 2016;24:4871-8. [Crossref] [PubMed]
- Chow E, Wu JS, Hoskin P, et al. International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiother Oncol 2002;64:275-80. [Crossref] [PubMed]
- Chow E, Nguyen J, Zhang L, et al. International field testing of the reliability and validity of the EORTC QLQ-BM22 module to assess health-related quality of life in patients with bone metastases. Cancer 2012;118:1457-65. [Crossref] [PubMed]
- Chow E, Hird A, Velikova G, et al. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for patients with bone metastases: the EORTC QLQ-BM22. Eur J Cancer 2009;45:1146-52. [Crossref] [PubMed]
- Groenvold M, Petersen MA, Aaronson NK, et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer 2006;42:55-64. [Crossref] [PubMed]
- Caissie A, Culleton S, Nguyen J, et al. EORTC QLQ-C15-PAL quality of life scores in patients with advanced cancer referred for palliative radiotherapy. Support Care Cancer 2012;20:841-8. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Chow E, Hoskin PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) SC 20. Clin Oncol (R Coll Radiol) 2006;18:125-8. [Crossref] [PubMed]
- Wong E, Chow E, Zhang L, et al. Factors influencing health related quality of life in cancer patients with bone metastases. J Palliat Med 2013;16:915-21. [Crossref] [PubMed]
- Püsküllüoğlu M, Zygulska AL, Tomaszewska IM, et al. Evaluation of health-related quality of life and its main influencing factors in a Polish population of patients with bone metastases. Curr Probl Cancer 2016;40:183-97. [Crossref] [PubMed]
- Montague L, Green CR. Cancer and breakthrough pain's impact on a diverse population. Pain Med 2009;10:549-61. [Crossref] [PubMed]


