Quality of life and symptom prevalence in children with cancer in Lebanon: the perspective of parents
Introduction
The life expectancy of children with cancer has increased dramatically due to early detection and innovations in treatment modalities (1). Yet, this population suffers from a great deal of physical and psychological symptoms like lack of energy, pain, nausea, worrying and sadness that are either cancer- or treatment related in etiology (2,3).
In 1998, the World Health Organization defined Pediatric Palliative Care (PPC) as ‘the active total care of the child’s body, mind and spirit, and also involves giving support to the family. It begins when illness is diagnosed, and continues regardless of whether or not a child receives treatment directed at the disease. Health providers must evaluate and alleviate a child’s physical, psychological, and social distress. Effective palliative care requires a broad multidisciplinary approach that includes the family and makes use of available community resources; it can be successfully implemented even if resources are limited. It can be provided in tertiary care facilities, in community health centers and even in children’s homes (4).
The introduction of PPC services has helped oncology patients in symptom control and in ameliorating the quality of their lives (5,6). Research studies have frequently utilized measurement tools like the Pediatric Quality of Life Inventory (PedsQL), the Memorial Symptom Assessment Scale (MSAS) or the Child Health Questionnaire in PC. In evaluating patients’ progress, clinicians and other healthcare professionals have focused on the self-report of children, proxy-parents or both (7-12). However, parent-proxy reports are considered vital; they provide comprehensive and complimentary outlook regarding their children’s quality of life (QoL) and symptom experience throughout the disease process.
The literature regarding symptom prevalence and management among cancer children from the perspective of parents is scarce. In a Canadian study, parents reported mood swings, fatigue and distress in missing social activities with friends/peers being highly prevalent among their children (13). In a Swedish study using the MSAS-parent’s version, lack of energy, pain and lack of appetite were found to be prevalent (9). Also, most of the studies assessing QoL in cancer children have addressed both the perspective of children and their parents at the same time. Based on the perception of parents, girls had significantly lower total QoL scores measured by the PedsQL cancer module compared to boys. Children with brain tumors were less worried and had more cognitive problems when compared to those with acute lymphoblastic leukemia (8). Tomlinson and colleagues found that cancer children at end of life had the worst physical health, pain and fatigue as reported by their parents (14).
So far, there are no studies in Lebanon describing the QoL and symptom management of cancer children based on the perception of the parents. A Lebanese study evaluated the quality of PC based on the perspective of bereaved parents and included the symptom experience of children at end-of-life (15). Though the study identified specific deficiencies in the management of dying children however a major limitation was recall bias. A phenomenological study focused on the lived experiences of 12 Lebanese parents while taking care of a child with cancer. Two of the major themes that emerged from the study were alterations in family QoL, and the impact of cancer on family members and sibling dynamics (16). Thus, the purpose of this study was to evaluate QoL, symptom prevalence and management, and quality of PC provided to Lebanese pediatric cancer patients from their parents’ perspectives.
Methods
Participants
The study adopted a cross-sectional descriptive survey design. Between 2010 and 2011, a convenience sample of parents of children with cancer was recruited from the Children’s Cancer Center of Lebanon (CCCL). This referral center that is located in the city of Beirut at the American University of Beirut Medical Center provides cancer treatments and PC to pediatric oncology patients from all over Lebanon and neighboring countries.
Parents and significant others directly responsible for the care of the children were included in the study. Inclusion criteria of parents included the following: having children 7-18 years of age, diagnosed for more than one month with cancer, receiving cancer treatments during the interview period, and residing in the country.
For ethical considerations, the study obtained Institutional Review Board and hospital administration approvals; written parental consents were obtained prior to the interviews.
During data collection, a trained research assistant (RA) approached the parents of children with cancer from both inpatient and outpatient units of the CCCL for possible enrollment. The study questionnaires were administered using face-to-face interviews in the private conference room of both units, during which the children were being attended by their health care providers. The RA read the questions, explained the scales, and documented their answers only. On average, the interview lasted around 40 minutes.
Instruments
The questionnaire is a combination of the PedsQL 3.0 Cancer Module (parent’s version), the MSAS, and the Needs at End of life Screening Tool (NEST), and a section on demographic and clinical characteristics.
The PedsQL 3.0 cancer module (parent’s version) consists of 27 items with 8 subscales assessing parental perceptions regarding their children’s health-related quality of life (HRQL) in the past one month. The instrument’s reliability and validity has been well documented in the literature (7,17,18). The subscales are pain and hurt (2 items), nausea (5 items), procedural anxiety (3 items), treatment anxiety (3 items), worry (3 items), cognitive problems (5 items), perceived physical performance (3 items), and communication (3 items). The items are rated on a 5-point Likert scale ranging from 0 (never a problem) to 4 (almost always a problem), reverse scored, and linearly transformed to scores from 0 (worst health) to 100 (best health). The scale scores represent the sum of the items divided by the number of the items corresponding to the scale, where higher scores indicate fewer problems/symptoms and better HRQL. A total cancer score represent the mean scores of the 8 subscales (7).
The MSAS 10-18 is an adapted self-rated instrument of 30 physical and psychological symptoms completed by pediatric oncology patients between 10 to 18 years of age regarding their symptom experience in the past one week. It has been validated in the pediatric cancer population with good psychometric properties (2). Prevalent symptoms are measured in terms of frequency, severity and distress, and a symptom score represents the mean of the three dimensions. The MSAS 10-18 is composed of three subscales: Physical (PHYS), Psychological (PSYCH) and the Global Distress Index (GDI). The PHYS subscale corresponds to mean symptom scores of 11 physical symptoms (lack of appetite, lack of energy, pain, feeling drowsy, constipation, dry mouth, nausea, vomiting, change in food taste, weight loss, and dizziness). The PSYCH subscale is computed as the mean symptom score of six psychological symptoms (feeling sad, feeling irritable, worrying, feeling nervous, difficulty sleeping, and difficulty concentrating). The GDI is calculated based on the mean of the frequency scores of four psychological symptoms (feeling sad, feeling irritable, worrying, and feeling nervous) and the distress scores of six physical symptoms (lack of appetite, lack of energy, pain, feeling drowsy, constipation, and dry mouth). In addition a total MSAS (TMSAS) is obtained as the mean of all the 30 symptom scale scores.
For this study, the MSAS 10-18 was modified in order to address the symptom experience of children from the perspective of their parents (9). The authors added an additional section to the instrument that addressed symptom management and treatment success (1= successful, 2= not successful).
From the Needs at End of life Screening Tool (NEST), the 23 selected items addressed areas related to financial burden (3 items), medical care (10 items), spirituality (6 items), and relationships (4 items) (19). The calculated scores represent the total mean of individual means of the items corresponding to the scales. The ratings range from 1 to 10; where higher scores (except finances) indicated better quality of the concept being measured. A low mean score on the financial burden scale indicate less monetary difficulties.
Data on demographics of parents and clinical characteristics of their children with cancer were obtained from the parents during the interview.
Translation and pilot study
The PedsQL 3.0 cancer module and MSAS were translated from their original English versions into Lebanese Arabic using the back-translation method. This method follows the process of forward and backward translations and ensures the acquisition of semantically equivalent instruments (20). After the initial forward translation into Arabic by the research team, the translated version was evaluated by two Arabic linguists; minor wording and grammatical changes were made. During the backward translation, a certified translator with no previous knowledge of the original instrument translated the Arabic version into English. Prior to initiating the pilot study, the final Arabic version was evaluated for cultural appropriateness by a team of experts; two in pediatric palliative care and two in research design and instrument development. The team recommended removing the third item (getting anxious about needle sticks; i.e.: injections, blood tests) from the procedural anxiety subscale in the PedsQL 3.0 cancer module because of its similarity in the Arabic language to the rest of the two items. Also, negatively worded items on the NEST were changed into positively worded items.
In the pilot study, four parents completed the final questionnaire and evaluated it on clarity, length, comprehension and presence of any difficult and bothersome questions. Based on the parents’ input, the Arabic wording of the symptoms feeling irritable and feeling drowsy were replaced with other Arabic synonyms for better understanding. No further changes were made.
Statistical analysis
For descriptive analyses of demographic and clinical characteristics, means, standard deviations (SD), frequencies (N), and percentages were used. The scores of the instruments (PedsQL 3.0 cancer module, MSAS 10-18, and NEST) were computed based on the instructions of the scoring manual (refer to instrument section). Independent sample t-test and analysis of variance (ANOVA) were used to evaluate differences in the total and mean subscale scores of the PedsQL3.0 cancer module and sample characteristics (relation to child, parent’s education, maternal employment, child’s age and gender, cancer type, days lost from school and elapsed time since diagnosis). A P value of ≤0.05 is considered statistically significant.
Data management and statistical analyses were done using SPSS 20.0 software.
Results
Sample characteristics
Between 2010 and 2011, a total of 85 parents were enrolled in the study with a response rate of 96.6%. Only 3 parents refused participation because they were preoccupied with preparing their children’s hospital admission papers. Mostly, the interviews were conducted in the outpatient clinic (82.4%) of the CCCL where pediatric patients receive their same-day cancer treatments.
Table 1 is a summary of demographic characteristics of parents. Most of the participants were mothers (68.2%), unemployed (77.6%) and had up to secondary or technical education (67.1%).
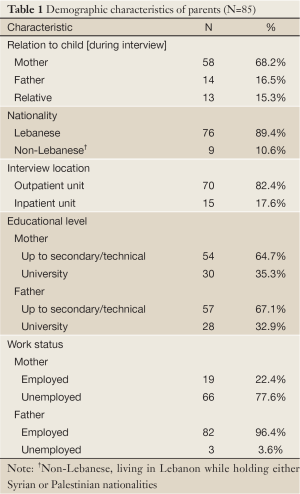
Full Table
Table 2 presents the biographical and clinical data of children with cancer. The children’s mean age was 12.55 years, females (51.8%), mostly having leukemia (44.7%), followed by lymphomas (21.2%) and head and neck cancer (14.1%). More than half of the sample (62.4%) was on chemotherapy and only 16 children (18.8%) had developed other medical conditions.
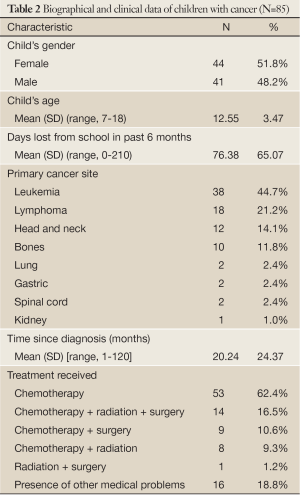
Full Table
PedsQL 3.0 subscales
The total cancer scale score was 72.75 (SD=15.47) indicating acceptable HRQL. Five of the eight subscale scores were greater than 70. The highest scores among the five subscales were communication (score=86.56; SD=20.77) and cognitive problems (score=80.47; SD=21.43). The lowest scores denoting more problems as viewed by the parents were found in nausea (score=55.22; SD=25.37), treatment anxiety (score=67.74; SD=30.17) and worry (score=68.62; SD=28.27) (Table 3).
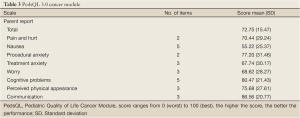
Full Table
MSAS 10-18
Based on parental input, children on average experienced 7.42 symptoms (SD=4.05) over a period of one week. The most prevalent symptoms were feeling irritable (57.6%), feeling nervous (56.5%), lack of energy (55.3%) and lack of appetite (49.4%). The highest symptom scores were found in difficulty swallowing (mean=2.44; SD=0.96), feeling sad and dry mouth (mean=2.33; SD=0.84), pain and “I don’t look like myself” (mean=2.33; SD=0.97) indicating higher frequency, more severity, and greater distress. The symptoms that were mostly addressed by the healthcare team were nausea (80.6%), vomiting (79.2%), and pain (67.5%) with treatment success rates ranging between 66.7% and 73.7%. As for the MSAS subscales, the PSYCH scores were the highest (mean=2.00; SD=0.70), followed by the TMSAS (mean=1.94; SD=0.50), PHYS (mean=1.93; SD=0.59) and GDI (mean=1.91; SD=0.74). Table 4 represents MSAS symptom prevalence and management in details.
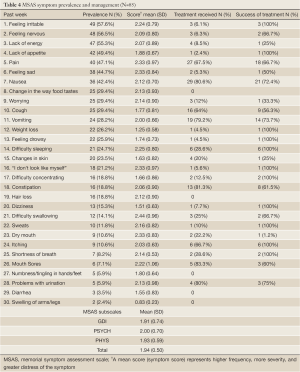
Full Table
Figure 1 is a visual representation of the most prevalent MSAS symptom mean scores.
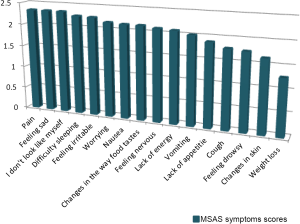
Among the 16 symptoms (prevalence greater than 20%), the highest mean scores were found equally in pain, feeling sad, and “I don’t look like myself” followed by feeling irritable and difficulty sleeping. Most of these symptoms despite being highly prevalent and psychological in nature did not receive any form of treatments.
Relationship between sample characteristics and PedsQL 3.0 subscales
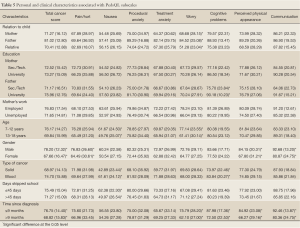
The relatives who took care of the children in the past month reported statistically lower worry scores compared to the mothers and fathers (51.28, SD=23.04 vs. 68.68, SD=28.15 and 84.52, SD=25.08) (Table 5).
Full Table
Fathers with university education viewed their children to have less cognitive problems compared to fathers with secondary or technical education (90.18, SD=10.23 vs. 75.70, SD=23.84).When compared based on children’s age, older children (13-18 years) were found by their parents to be significantly more worried than younger children (8-12 years) (61.41, SD=30.14 vs. 77.14, SD=23.55). Also, females had significantly lower scores on total cancer scale, pain and hurt, perceived physical appearance and communication subscales compared to the males (P<0.05). Children with solid tumors had statistically significant lower scores on the nausea and cognitive problems subscales than those with blood cancer. In addition, children skipping school for more than 45 days had more problems with nausea than those who skipped less from school (49.97, SD=26.54 vs. 62.38, SD=22.30). A comparison based on elapsed time since diagnosis, showed that children living with cancer for more than 9 months had significantly lower total cancer score (68.82, SD=15.83), were more worried and had cognitive, perceived physical appearance and communication problems compared to the group who are diagnosed with cancer for less than 9 months (P<0.05) (Table 5).
NEST
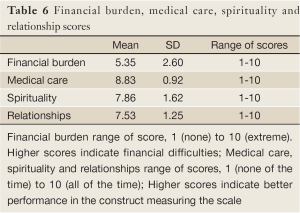
The financial burden scale score was 5.35 (SD=2.60) indicating that parents had less financial hardship during their children’s illness. The medical care scale had a score of 8.83 (SD=0.92), followed by the spirituality (score=7.86; SD=1.62) and the relationships (score=7.53; SD=1.25) scales. It is worth mentioning that the item “you try to help those around you prepare for the possibility of losing your child” in the relationships scale scored a low mean of 3.21 (SD=3.29). This finding indicates that parents most of the time did not prepare their surrounding for their child’s possible loss (Table 6).
Full Table
Discussion
To our best knowledge, this is the first study in Lebanon assessing parental perceptions regarding the QoL and symptom prevalence, in addition to the quality of care provided to a heterogeneous sample of children diagnosed with different types of cancer. The results in our study showed that children with cancer had treatment anxiety, more nausea, and greater worry as perceived by their parents. When compared to Varni et al.’s parent-proxy report in the original study, children faced more problems in procedural anxiety only (7). Our findings were to great extent similar to a Chinese study, where parents reported their children who were receiving cancer treatments to have more problems in nausea, worry, treatment anxiety, and procedural anxiety (21).
The total cancer score in our study was 72.75 (SD=15.47) indicating that children based on their parent’s standpoint had overall acceptable HRQL. A similar total score of 74.91 (SD=15.25) was reported by parents from Japan (18). When the PedsQL total score was compared based on gender, females had significantly a lower mean score that was in line with a study from the United States (8). As for the subscales scores, girls experienced significantly more pain and hurt and had perceived physical appearance problems when compared to the boys in our sample.
When the total and subscale scores were compared based on time since diagnosis, children diagnosed with cancer for more than 9 months had significantly lower mean scores in the total and four of the subscales; worry, cognitive problems, perceived physical appearance and communication. The children living longer with the disease (>9 months) were significantly more worried and faced issues with physical appearance too (suboptimal QoL scores <70). The significance of being worried was also common in a Canadian study by Tomlinson and colleagues reported by parents, where older children and those living longer with cancer had the lowest scores (more worry) (14). In our Lebanese sample, a comparison based on age showed that adolescents (13-18 years) were more worried (P<0.05) in addition to suffering from nausea.
Based on the MSAS, parents on average reported 7.42 symptoms with feeling irritable and nervous, lack of energy, lack of appetite and pain being most frequent among their children. A longitudinal Swedish study by Poder and colleagues based on parents’ perceptions reported to greater extent similar results. Children after four months of being diagnosed with cancer had around 8.9 symptoms with pain, lack of energy and lack of appetite being highly prevalent (9). Despite using different symptom assessment tools, bereaved parents from Lebanon (15) and Australia (22) identified fatigue, anorexia, and pain to be the most frequent symptoms experienced by children at the end of their lives. When our findings were compared to an unpublished data on symptom prevalence reported by Lebanese children with cancer, the results were similar to a greater extent. The younger generation (aged 7-12 years) mostly had appetite loss, pain and nausea, while older children (aged 13-18 years) complained from fatigue, being irritable, pain, worrying, and lack of appetite (23). Similarly and based on patients’ reports, Collins et al. (2) found a group of physical (lack of energy, pain, drowsiness, nausea, cough and lack of appetite) and psychological symptoms (feeling sad and nervous, worrying and feeling irritable) to be predominant among cancer children with prevalence rates of greater than 35%. Miller and colleagues reported nausea, fatigue, lack of appetite, pain and feeling drowsy being mostly common among hospitalized oncology patients between 10 to 17 years of age (24).
As for the MSAS subscale scores, our results were higher than those reported by the Swedish sample that measured these subscales at three different intervals over a period of four months (9). Moreover, Lebanese parents viewed the psychological symptoms to be more distressing and troublesome on their children than the physical symptoms. Interestingly, a Canadian study addressing family caregivers’ perceptions in adult cancer patients reported higher psychological symptom scores too (25). A probable explanation in our results (PSYCH=2.00; SD=0.70) may be related to the fact that the psychological symptoms were poorly addressed by the healthcare team in the center.
In symptom management, parents reported that the most treated symptoms were nausea, vomiting, pain, and cough with treatment success ranging between 56.3% and 73.7%. A visual representation (Figure 1) of the mean symptom scores in top 20% of prevalent symptoms showed that pain, feeling sad, and “I don’t look like myself” to be the most burdensome; interventions however were geared towards pain management and in relieving nausea/vomiting. When compared to the feedback of Australian parents in a study by Heath and colleagues, our results in treatment success were higher in pain and lower in fatigue and poor appetite (22). In the Lebanese study of bereaved parents, pain and dyspnea were mostly treated in children with treatment being successful in 42.1% and 55.5% respectively (15). Interestingly, the results of an unpublished data on symptom management as reported by Lebanese pediatric cancer patients showed that medications administered to treat nausea and pain were the most successful ranging respectively between 77.8% and 80.0%. Adolescents reported that the treatments for nausea, pain, cough and vomiting were mostly successful (>66.0%) (23).
A final note on symptom management, our results (Table 4) based on the perspective of parents are suggestive that PPC professionals need to treat not only the physical but psychological symptoms in order to alleviate unnecessary suffering. In addition more emphasis is warranted to address less prevalent but highly distressing symptoms like difficulty swallowing that had the highest mean symptom score in our study.
The financial burden of parents during their children’s disease process was average (score=5.35; SD=2.60). This finding is due to the fact that all medical and hospitalization expenses of pediatric oncology patients were either covered by CCCL, or by various types of health insurance agencies. However, parents expressed facing some personal monetary difficulties that was not directly related to the care but as a result of unemployment or skipping more work days in order to be with their children during the hospital visits. In a Lebanese phenomenological study by Khoury and colleagues on parents’ experiences living with a child with cancer, the theme “living with added burdens” such as financial difficulties and additional parental responsibilities emerged during the interviews (16). Another qualitative study conducted in the United States addressing family caregiver burden while taking care of patients with brain tumor found that families often encountered physical, social, emotional, and financial challenges despite providing good quality care to their loved ones (26).
It is noteworthy to address the financial hardship of patients and families because of high medical fees and expensive oncologic treatments that is a common and major problem in Lebanon. Unfortunately, many Lebanese patients are neither privately insured nor eligible for the Ministry of Health National Social Security Fund in attaining their cancer care medications, thus self-pay all expenses that cause immense financial difficulties and ultimately contribute to delays in their recovery.
In our study, most of the Lebanese parents were greatly satisfied with the quality of care provided by the CCCL’s oncologists and nurses; which was in concordance with a previous Lebanese study by Saad and colleagues on the bereaved parental evaluation of the palliative care program provided by the same center (15). Also, a high medical score of 8.83 (Table 6) can be attributed to the center’s healthcare delivery system, the easy accessibility of healthcare services, and the close monitoring and follow-ups. Furthermore, parents accredited their satisfaction to the adopted caring approach by the team and their participation in making decisions. Our findings are in line with Wolfe et al. evaluating the quality of palliative care from parent’s own perspectives. Parents rated the overall quality of care as very good or excellent provided by the oncologists, primary care nurses, and the psychosocial clinicians (27).
When assessing for children’s relationships and spirituality domains, the scores were similar and above average. These high results emphasize the role of religion and family ties that are strong support systems in the Lebanese society. As reported by our Lebanese parents, the children often had close network of friends and family members to rely on, play with, and confide in them their problems. Contrary to our finding, parents in the Lebanese study by Khoury and colleagues frequently encountered sibling rivalry and disrupted sibling dynamics because of their children’s illness (16). As for religion, the parents in our sample reported that their children continued to experience spiritual growth, prayed and had positive outlook towards the future. The latter was further emphasized where parents rarely prepared the surrounding for the possibility of losing a child to cancer. The supportive role of religion and the optimistic approach towards a better future was reported by the two Lebanese studies on the lived experiences of families living with a child or an adult with cancer (16,28).
Limitations
There are a number of limitations that needs to be addressed in this study. One possible limitation is related to the cross-sectional design that does not allow a complete evaluation of children’s QoL and symptom experience changes at different time intervals throughout the disease process by their parents. Another possible limitation that may influence the interpretation of our results is selection bias. Around 83% of the parents were recruited from the outpatient clinic that may not reflect the experiences of parents with hospitalized children. In addition, the authors are cautious in generalizing the current findings that is based on the parents’ perspectives to the overall Lebanese pediatric oncology population. The reason is related to the fact that data was collected from the only referral center in the country that is specialized in PPC, and where treatment modalities may differ or are less accessible in other hospitals.
Conclusions
In summary, the parents’ input regarding HRQL and symptom management highlighted the problematic areas that need be addressed carefully by PPC teams during cancer treatments.
Although children had overall acceptable QoL according to their parents, however they were anxious in receiving treatments and worried about their recovery. Moreover, the comprehensive evaluation of patients in symptom prevalence and treatment emphasizes the need for more effective assessment and treatment of the psychological as well as the physical symptoms that jeopardize QoL.
As a final note, we recommend conducting further national studies from multiple medical centers that focus on child self-and parent-proxy reports in evaluating QoL and symptom experience among Lebanese children with cancer.
Acknowledgements
The authors would like to thank the parents of children with cancer for their participation. Likewise, we are grateful to the Lebanese National Council for Scientific Research for funding this study.
Disclosure: The authors have no disclosures or conflicts of interest. The submitted manuscript is not published or in review elsewhere.
References
- American Cancer Society. Cancer facts and figures 2012. Atlanta GA, USA, 2012.
- Collins JJ, Byrnes ME, Dunkel IJ, et al. The measurement of symptoms in children with cancer. J Pain Symptom Manage 2000;2:363-77. [PubMed]
- Collins JJ, Devine TD, Dick GS, et al. The measurement of symptoms in young children with cancer: The validation of the Memorial Symptom Assessment Scale in children aged 7-12. J Pain Symptom Manage 2002;2:10-6. [PubMed]
- World Health Organization (WHO). WHO definition of palliative care for children. 1998. [cited 2013 Jan 17]. Available online: http: //www.who.int/cancer/palliative/definition/en/
- Mack JW, Wolfe J. Early integration of pediatric palliative care: for some children, palliative care starts at diagnosis. Curr Opin Pediatr 2006;2:10-4. [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;3:733-42. [PubMed]
- Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002;2:2090-106. [PubMed]
- Meeske K, Katz ER, Palmer SN, et al. Parent proxy-reported health-related quality of life and fatigue in pediatric patients diagnosed with brain tumors and acute lymphoblastic leukemia. Cancer 2004;3:2116-25. [PubMed]
- Pöder U, Ljungman G, von Essen L.. Parents’ perceptions of their children’s cancer-related symptoms during treatment: a prospective, longitudinal study. J Pain Symptom Manage 2010;2:661-70. [PubMed]
- Walker AJ, Gedaly-Duff V, Miaskowski C, et al. Differences in symptom occurrence, frequency, intensity, and distress in adolescents prior to and one week after the administration of chemotherapy. J Pediatr Oncol Nurs 2010;2:259-65. [PubMed]
- Levi RB, Drotar D. Health-related quality of life in childhood cancer: Discrepancy in parent-child reports. Int J Cancer Suppl 1999;2:58-64. [PubMed]
- Sawyer M, Antoniou G, Toogood I, et al. A comparison of parent and adolescent reports describing the health-related quality of life of adolescents treated for cancer. Int J Cancer Suppl 1999;2:39-45. [PubMed]
- Dupuis LL, Milne-Wren C, Cassidy M, et al. Symptom assessment in children receiving cancer therapy: the parents’ perspective. Support Care Cancer 2010;2:281-99. [PubMed]
- Tomlinson D, Hinds PS, Bartels U, et al. Parent reports of quality of life for pediatric patients with cancer with no realistic chance of cure. J Clin Oncol 2011;2:639-45. [PubMed]
- Saad R, Huijer HA, Noureddine S, et al. Bereaved parental evaluation of the quality of palliative care program in Lebanon. Pediatr Blood Cancer 2011;2:310-6. [PubMed]
- Khoury MN, Huijer HA, Doumit MA. Lebanese parents’ experiences with a child with cancer. Eur J Oncol Nurs 2013;2:16-21. [PubMed]
- Tanir MK, Kuguoglu S. Turkish validity and reliability of a Pediatric Quality of Life cancer module for children aged 8-12 and parents. Asian Pac J Cancer Prev 2011;2:125-30. [PubMed]
- Tsuji N, Kakee N, Ishida Y, et al. Validation of the Japanese version of the Pediatric Quality of Life Inventory (PedsQL) cancer module. Health Qual Life Outcomes 2011;1:22. [PubMed]
- Emanuel LL, Alpert HR, Baldwin DC, et al. What terminally ill patients care about: toward a validated construct of patients’ perspectives. J Palliat Med 2000;1:419-31. [PubMed]
- Varricchio CG. Measurements issues concerning linguistic translations. Sudbury, MA: Jones and Barlett Publishers; 2004.
- Ji Y, Chen S, Li K, et al. Measuring health-related quality of life in children with cancer living in Mainland China: feasibility, reliability and validity of the Chinese Mandarin version of PedsQL 4.0 Generic Core Scales and 3.0 Cancer Module. Health Qual Life Outcomes 2011;1:103. [PubMed]
- Heath JA, Clarke NE, Donath SM, et al. Symptoms and suffering at the end of life in children with cancer: an Australian perspective. Med J Aust 2010;3:71-5. [PubMed]
- Abu-Saad Huijer H, Sagherian K, Tamim H. Quality of life and symptom prevalence among pediatric cancer patients in Lebanon. American University of Beirut, Hariri School of Nursing. [Unpublished manuscript].
- Miller E, Jacob E, Hockenberry MJ. Nausea, pain, fatigue, and multiple symptoms in hospitalized children with cancer. Oncol Nurs Forum 2011;2:E382-93. [PubMed]
- Lobchuk MM. The Memorial Symptom Assessment Scale: Modified for use in understanding family caregivers’ perceptions of cancer patients’ symptom experiences. J Pain Symptom Manage 2003;2:644-54. [PubMed]
- Schubart JR, Kinzie MB, Farace E. Caring for the brain tumor patient: Family caregiver burden and unmet needs. Neuro Oncol 2008;2:61-72. [PubMed]
- Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med 2000;3:326-33. [PubMed]
- Doumit MA, Huijer HA, Kelley JH, et al. The lived experience of Lebanese family caregivers of cancer patients. Cancer Nurs 2008;2:E36-42. [PubMed]


