Challenges and ethical issues in the course of palliative care management for people living with advanced neurologic diseases
Introduction
The Institute of Medicine defined palliative care (PC) in their recent report as “care that provides relief from pain and other symptoms, that supports quality of life (QOL), and that is focused on patients with serious advanced illness and their families” (1). While the World Health Organization defined it as “an approach that improves the QOL of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual” (2). In line with patient- and family-centered care, the Clinical Practice Guidelines for Quality Palliative Care defined PC as “patient and family-centered care that optimizes QOL by anticipating, preventing, and treating suffering” (3). A commonly used term interchangeably with PC is hospice which offers a “comprehensive, socially supportive, pain-reducing and comforting alternative to technologically elaborate, medically centered interventions” and is a way by which PC needs of patients at the terminal stage of their illness are delivered (1,4).Traditionally, PC has been utilized among cancer patients however it’s concepts in management have been applied as early as the late 1980s with the American Academy of Neurology’s (AAN’s) position statement in the management of a persistent vegetative state patient (5). Subsequently, the AAN section on Pain and PC was formed in 1995 to improve on the PC involvement in neurological disorders. PC is important in the management neurological disorders, as very often, these conditions are debilitating, often irreversible, and have significant physical and emotional impact on the patients and their families. Due to the prolonged, fluctuating and unexpected course of some neurologic disease, accompanied by progressive functional loss of mobility, communication ability and cognition, all of which affects the patient and caregivers, PC should be considered an important part in caring for patients with neurological conditions both in the inpatient or outpatient setting (6). In 1996, the need to incorporate PC in the care for patients with neurologic conditions was recognized by the AAN Ethics and Humanities subcommittee (7,8). In this statement, the duties of neurologists to provide adequate PC and improve education on PC were promoted. The European Academy of Neurology in collaboration with European Association for PC developed recommendations which included early PC integration, incorporation of a multidisciplinary team, open and structured communication with patient and family or caregiver, proactive symptom assessment and management, caregiver support, end-of-life care recognition, assessment and management and training and education of neurologists (9). As the patient goes through the process from diagnosis to the end-stages of their neurological disease, challenges and ethical issues may arise even when the patient is already under palliative level of care. Ethical principles developed by Beauchamp and Childress of respect for autonomy, beneficence, non-maleficence and justice (10) need to be consistently examined.
In this article, we reviewed and summarized the challenges and ethical issues in the context of PC management of patients with advanced acute, rapidly progressive, slowly-progressive or degenerative neurological conditions that are commonly encountered in practice. Acute neurologic conditions included cerebrovascular disorders and vegetative state. Rapidly progressive condition of Creutzfeldt-Jakob disease (CJD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease and other PD-related disorders, Alzheimer’s disease (AD) and other dementias and central nervous system (CNS) malignancies and other tumors are included in the slowly-progressive or degenerative disorders although some CNS malignancies may rapidly progress. Common challenges and ethical dilemmas across all the categories are presented. We also presented neurologic disease-specific considerations in the course of PC management.
Ethical dilemmas across neurologic diseases
Timing of PC involvement
There is currently no general guideline on the timing of PC for neurologic disease; however, disease-specific recommendations are emerging. A distinction between a neurologist with the interdisciplinary team applying PC approach (primary PC) and consulting a specialized palliative care team (specialist PC) should be made. The AAN practice parameter in ALS recommended multidisciplinary team involvement inpatient management which included PC for symptom management most especially in the terminal stages (11); while for stroke, the initial PC management more often comes from primary PC (4,12). The American Heart Association and American Stroke Association however recommends that for more complicated symptom management and decision making, specialty palliative care (SPC) should be involved (4,12). For patients with neurocritical illness, it is recommended that specialist PC be involved earlier in the stage of the disease to establish rapport and trust with the family however, their involvement should not discount the concomitant and continuous primary PC management from the primary neuro-intensive care interdisciplinary team (13). An emerging model of PC particularly applicable in neurologic conditions is “simultaneous care” (14,15) where in early PC integration is done in the disease and throughout the disease process with the aim of improving the quality of care through symptom control, strengthening the support structure to improve patient and caregivers’ quality of life and facilitating appropriate resources needed (16). With this approach, PC management is encouraged at the moment of diagnosis (17). Until further prospective studies reveal an optimal disease-specific timing for involvement of PC, applying primary PC and considering an individualized approach to the timing of utilizing SPC is still recommended.
Surrogate decision making and the “Unbefriended” patient
It is common among patients with neurologic disease to have impaired cognition or communication. Both of these affect decision-making capacity (DMC) as it pertains to laws in the United States. The ability to be able to exercise this capacity involve the patient being able to understand the current condition, the testing needed, available management options and the result of not proceeding with testing or management (18,19). The patient must be able to make a decision incorporating the information provided and his or her values and he or she should be able to clearly communicate the decision to the physician (18,19). If the patient lacks DMC, formally appointed surrogates through physicians or lawyers assigning a health-care proxy or durable power of attorney (DPOA) may make decisions in the patient’s best interest (20). In a cohort of 129 patients with neurologic conditions, only 23% of them had a Durable power of attorney (DPOA) appointed prior to the hospitalization (21) which is consistent with another report that only 25% of adults in the United States have advanced directives (22,23). If there is no available legal health-care proxy or DPOA, surrogate consent laws in some states may allow physicians to discuss decisions with an individual or group of people who can potentially express the patient’s wishes (24,25). The surrogate consent laws may be based on hierarchy or consensus (25). Among the 44 states that adopted surrogate consent laws two states (Colorado and Hawaii) utilize the consensus statutes requiring all available “interested persons” to have a unified consensus about who will be the decision maker (25). An example of surrogate law based on hierarchy is what is used in Georgia where in legal authority lies with several potential people in the following order spouse, parent of a minor, legal guardian, adult child, parent of an adult child, sibling, grandparent, adult grandchild, adult niece, nephew, aunt or uncle and adult friend (18). In the cases of the “Unbefriended Patients”, who are not able to exercise DMC and are alone (26,27), ethics consult are usually warranted according to the American Medical Association (27). While the American Geriatrics Society places the decision making role to the treatment team (28), a court appointed-guardian is what is recommended by the American College of Physicians (29). Institution and state laws vary in terms of patient management in these scenarios (29). In most cases the family makes a united decision, which is acceptable. If the family is unable to agree, a legal process by which a judge assigns a legal guardian or a surrogate may be done. In cases where the patient is under the care of the state, the state serves as the guardian and decision making is done through the state representative. Shared decision-making, the process by which the patient with or without other family members or friends and the health care team share information regarding treatment options, risks and benefits while the patient talks about their preferences according to their beliefs and values, is now a common practice (30).
The role and the process by which the surrogates should approach decision making for the patient should be clearly explained. A common mistake done by inexperienced physicians is asking surrogates “what do you want to do?” The ethical standards by which the surrogates’ decisions are made should be based primarily on, the patient’s known wishes, followed by substituted judgment and patients’ best interest (24,31). When the patient’s wishes are completely unknown, the surrogate should make their best assessment of what the patient may decide under the current situation from how best they know the patient, which is the standard of substituted judgment (32). It is however, important to note, that this has been an area of debate among bioethicists because there is some doubt into how reliable surrogates can be in terms of knowing what the patient would have wanted (33). In cases where in the surrogates are not able to utilize substituted judgment, the best interest standard based on established norms should be used in addition to discussion and analyzing the risk and benefits of the current treatment (33).
Withholding and withdrawing treatment
There is neither difference nor legal basis for distinguishing withholding treatment that is life-sustaining versus discontinuing treatment if the patient or the surrogate wishes to do so (20). In neurological conditions there are available prognostic tools that may aid in prognostication and guide the decision to withhold or withdraw life-sustaining treatment (WLST) however; other factors should still be considered before the decision is made such as the patient and family’s values, beliefs and current emotional and psychological states. For cardiac arrest, anoxic brain injury in itself do not lead to death as long as supportive ICU care is maintained (34). Death after cardiac arrest occurs in 60–90% after WLST (35-37). The concept of self-fulfilling prophecy, a “prediction that directly or indirectly causes itself to become true” also plays a role in most severe acute brain injuries (34). Current recommendations in terms of timing of prognostication, which may in turn affect the timing of WLST is to wait at least 72 hours especially in post anoxic brain injury (38). A retrospective study on intracerebral hemorrhage patients for which WLST was done, compared the median predicted probability of 1-year death and severe disability against the decision to WLST to determine if practitioners were prone to self-fulfilling prophecies when managing ICH (which included subarachnoid, subdural hematoma and intraparenchymal hemorrhage). They found that the patients who underwent WLST still would have died based on probability models which show that in ICH, it is unlikely that WLST lead to a self-fulfilling prophecy (39). It is best to avoid early WLST which has been defined as discontinuation of life sustaining therapy before 72 hours (40) especially in patients with neurologic conditions with an unpredictable course since prediction scores for several neurologic conditions vary in their accuracy of prediction of death and disability (41-46). For patients with acute onset severe neurologic conditions especially in post-cardiac arrest patients with anoxic brain injury, although early PC involvement is encouraged, it is recommended to not transition to WLST until at least after 72 hours (47-49).
Pain management, palliative sedation (PS) and the principle of double effect
Most commonly, therapy to alleviate pain and discomfort for patients that transitioned to palliative level of care also have adverse effects which most commonly involve respiratory depression and further decrease in level of alertness in the setting of opioid or benzodiazepine use. The principle of double effect is applicable when one uses therapy with an intended beneficial effect but with an unintended foreseen harmful effect (50,51). It is important to note that comorbid pulmonary conditions that may be present in these patients may affect the response of these patients to opioids or sedation in such a way that respiratory depression may occur either in a faster rate or at a more severe degree compared to a patient without pulmonary issues. The physician’s act of administering opioids is not intrinsically wrong with an intended good effect even if an adverse effect may have been anticipated however legal fears still may limit its administration (52). To apply the criteria of the Principle of Double effect in this setting the following conditions should be present: the action from which harm results is good, the intention or motivation must be sincerely good and the harmful effect is not intended, the harmful effect must be immediately due to the good effect and the proportion of the reason for the good effect should be serious enough to allow for the side-effect to occur (53-55). The principle of Double Effect also applies to PS which has been defined as the “intentional reduction of vigilance by pharmacological means up to the point of the complete loss of consciousness with the aim of reducing or abolishing the perception of a symptom that otherwise would be intolerable for the patient despite the implementation of the most adequate means aimed at controlling the symptom itself, which is therefore to be considered refractory” (56). It has not been shown that sedated patients’ survival differs from those not sedated in the terminal phase of their condition (57-63). Indications include dyspnea, delirium (62,64-67), pain, massive bleeding or intractable vomiting (64,68) all of which are applicable to patients with neurologic conditions. Once control of symptoms is achieved, up titration of the medications should stop (69). A survey among neurologists showed agreement that at the end stage of their disease, the practice of sedation for the terminally ill is acceptable when it was considered refractory to other interventions. However, its use on patients in the earlier stages of their disease and are not imminently dying is still controversial (64).
The use of neuromuscular blockade
Although the use of neuromuscular blockers in the intensive care unit is prevalent, most especially for management of acute respiratory failure, status asthmatics, intra-abdominal or intracranial pressure control (70), its role in PC management has not been widely studied. Even with the intent of decreasing agitation manifesting as violent extremity or truncal movement, paralyzing a patient may mask pain or other symptoms of discomfort (71) especially in patients with primary neurologic dysfunction where in their sole means of expression of pain are movements. It has been proposed however that neuromuscular blockers may be used for patients with persistent agonal respiration in spite of adequate sedation (72). These agonal breaths may be seen in patients with spastic neuromuscular conditions, or central hyperventilation syndromes due to acute brain injuries. Because of the present uncertainty on the association of gasping and the patients’ level of pain or discomfort during this period, the principle of “maximin rule” may be applied where in a situation of uncertainty, one studies what is the worst possible scenario and what is the appropriate action that will avoid the worst case scenario (72,73). The use of neuromuscular blockers still continues to be debated and is limited and should be further studied due to the sparse availability of data.
Initiating, withholding or discontinuation of artificial nutrition and hydration
Artificial Nutrition includes oral nutritional supplements (ONS), enteral nutrition (EN) delivered through nasogastric (NG), nasogastrojejunal (NGJ), dobhoff tubes (DHT), percutaneous endoscopic gastrostomy (PEG) or jejunostomy (PEG-J) or surgical gastrostomy tubes (GT) or parenteral nutrition delivered via peripheral versus central venous lines. Artificial hydration includes water or electrolyte solutions through feeding tubes or parenteral means (74). It has been advocated by the American Association of Hospice and Palliative Medicine and the Hospice and Palliative Nurses Association that AHN in terminal stages of disease may cause an increased risk of infections, pressure sores, diarrhea and fluid overload (75,76) supporting withholding AHN during this period. It does not offer benefit during the terminal phase of illness and it has the potential to lead to discomfort especially if feeding tubes are in place, due to the likelihood of requiring restraints for the patient awake (77-80). If able, the patient may have Oral Assisted Comfort Feeding or “food for pleasure” where the goal of adequate nutrition is no longer paramount (81). It enables the patient to once again be part of a familiar social scenario where they are in the table eating with family (82). Patients with stroke, ALS, dementia or other neurologic disorders that allows for the patient to maintain truncal stability are ideal for this routine. The role of the speech and language therapists could not be underestimated as they will assess the likelihood of aspiration to establish expectations for the family. Food modification may be required such as adding thickeners or chopping food into smaller pieces (81). Another option is positioning the patients in their room and feeding them their while the family visits. As needed fluids may be given if the patient expresses thirst (69). Mild fluid administration to avoid excessive dehydration that may contribute to delirium and metabolic intoxication is an option (83,84). For those patients who are in their final stages, a common scenario in the neuro-intensive care unit, administration of intravenous fluids or enteral feeding may result in anasarca, diarrhea and increased secretions that contradict the goals of PC (81,85). This should be clearly explained to the families to avoid unnecessary distress regarding thoughts that the patient is “being starved to death”. Food for pleasure may be continued for as long as the patient is able to without causing discomfort, with the family knowing and understanding the goals, expectations and possible outcomes if oral intake is continued at this stage. In most state laws, the right to refuse artificial hydration and nutrition (AHN) may be relinquished to a surrogate. However, in several states, legislation has been introduced which assumes that a patient in persistent vegetative state would want to continue to receive ANH and if one desires to negate this assumption in court, discontinuation of ANH may only be done when one of the following specific conditions apply: inability to administer ANH, ANH administration would hasten death, ANH cannot be absorbed, there is a specific advance directive authorizing withdrawal applicable to the current clinical condition or if there is clear evidence that the patient expressed and gave informed consent to withdraw ANH as applicable to the current condition (86). Further prospective studies are warranted to delineate limits in ANH, assess its impact on the family and patient and on healthcare economics, and to analyze the impact of different state laws regarding AHN.
Renal replacement therapy in acute brain injury
Patients on long term dialysis represent a population of patients at risk for acute neurologic injuries including acute ischemic stroke (AIS), intracerebral hemorrhages (ICH), subarachnoid hemorrhage (SAH) and subdural hemorrhage (SDH) (87-90). They are also the same group of people that have a higher risk cardiovascular disease (89,91) that put them at risk of cardiac events that may lead to anoxic brain injuries. There are also patients with brain injury that develop acute kidney injury requiring dialysis in which decisions regarding the utility of dialysis may be in question. In these settings, recommendations from the Renal Physician Association regarding initiating or withdrawing dialysis may be utilized (92). Their most recent published update includes a prognostic model, discussion on the need to assess the patient for DMC and other treatment options and goals for these patients in consideration of their overall prognosis, situation, functional status and personal values (92). Several prognostic scoring systems may aid goals of care discussion for patients on dialysis with neurologic disorders such as iScore, PLAN score and ASTRAL Score in AIS (93); the ICH score and FUNC score for ICH (94,95) and the ALS-SS score for patients with ALS (96). Profound neurologic impairment where in the patient does not have awareness, sensation, purposeful movement or thought process is a situation where dialysis may be withheld or withdrawn (92). There may be a situation that a “time-limited trial” (97) is applicable where dialysis may be withdrawn if clinical improvement does not occur (92). This trial should be explained in terms of goals, outcome measured and duration prior to initiation to draw expectations and not cause further confusion (97,98). Utilization of this guideline, although not universal, is considered standard of care among most nephrologists (99). Further studies on how this guideline is used in the acute and long-term management of patients with neurologic disease both the inpatient and outpatient settings are needed.
Defibrillators in acute brain injury
Patients with congestive heart failure or arrhythmias that require them to have implantable cardioverter defibrillators (ICDs) and cardiac resynchronization devices with a pacemaker or defibrillator (CRT-P/CRT-D) are at risk for acute neurologic injuries such as stroke, anoxic brain injury, ICH for those who are on anticoagulation or traumatic brain injuries for those who suffer a fall due to malfunction of these devices. The approach between the conscious and unconscious differs. The ethical principle of autonomy should be applied as well as the laws of surrogacy for those who are not cognitively intact, which is the more common scenario among patients with neurologic disorders under palliative level care. The reason behind disabling implantable devices such as CRT is that it may activate a shock at the end-stage of the patient’s disease that may cause pain, distress, anxiety and decreased quality of life (100,101). Discontinuing a device that acts as a pacemaker or an inotrope may pose as a challenge because stopping these may actually cause death however, it has been recommended to manage these situations just like how one would manage discontinuation of mechanical ventilation for a patient with respiratory failure (101) in that the patient still goes through the natural course of death but the discontinuation of mechanical ventilation is not considered as the direct cause of death. Discussion on deactivation should follow the thorough process of informed consent where options are weighed with risk and benefits. When the decision is made to deactivate the device, a do-not-resuscitate (DNR) status should also be agreed upon (101). Further prospective studies are warranted to gain more information regarding patients with neurological disorders and implantable cardiac devices and how it impacts PC management.
Donation after cardiac death
Patients in the neuro-intensive care unit who don’t meet brain death criteria may still be able to donate organs through the process of “donation after cardiac death” or “DCD” (13). Even if the families would want to transition to PC, early involvement of organ procurement organization (OPO) will enable the family to consider this option, especially if the patient is a registered organ donor. It is essential however that organ donation is not brought up by any member of the primary team. Organ donation conversations require specific wording due to the sensitivity and implications of the process. Some members of the primary team may not be familiar with the process and how to answer questions and may cause miscommunication and distress on the family. The decision making on WLST should not be dependent on DCD or vice versa (13) although the involvement of a multidisciplinary team including the OPO staff and PC specialist is encouraged throughout the process to offer support and answer questions. Depending on the institution, immediate family members may be allowed in the surgical area to witness terminal extubation. The family should be informed that if cardiac death does not occur usually within 60 minutes of extubation, the patient is no longer a suitable donor and will be returned to the ICU for further end-of-life care (102) for which symptom control ensues to make the patient comfortable.
Seizures and status epilepticus
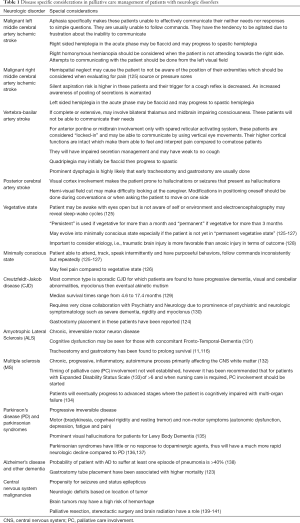
Patients at risk for seizures and status epilepticus include those with primary intracranial pathologies such as AIS, ICH, CNS malignancies, CNS infection or anoxic brain injury. Seizures are a “transient occurrence of signs or symptoms due to abnormal excessive or synchronous neuronal activity in the brain” (103). Status epilepticus is defined as seizures longer than 5 minutes or recurrent seizures without return to baseline (104) while refractory status epilepticus is status epilepticus that require further treatment after not responding to first- and second-line therapy of appropriate doses (104). Seizures may involve only a part or parts of the body while the patient maintains consciousness (focal or partial) or may involve the whole body impairing consciousness (generalized). Seizures may also start partial then progresses to being generalized (partial seizure with secondary generalization). More importantly, they are also classified according to the presence or absence of actual convulsions (Convulsive versus Non-Convulsive). Note that a patient that is in generalized convulsive status epilepticus may progress to a non-convulsive status epilepticus (NCSE) when only subtle twitches are noticeable due to the inability of the body to create strong muscle movements (104) or NCSE may present as delirium or change in mental status (105). The incidence of SE in the PC setting is not well established (106). Once transitioned to palliative level of care, there are limits imposed by the goals of care that will affect diagnostic and therapeutic management that should be discussed with the family especially since the available diagnostic and therapeutic algorithms available do not consider patient under PC (106,107) and the goals of PC largely differs from the curative goal of established guidelines. It is important however to delineate these limits as some families may want to still restore pre-SE baseline which will affect the management of SE (108). Anti-epileptic drugs (AED) should be continued in patients who are already on AEDs prior to transition to palliative level of care as it has been shown in a cohort of patients with high grade glioma that 35% of the patients for which it was tapered, seizures did occur (109) and this may cause difficulty in symptom management. This may be a common scenario for CNS malignancies (109) or those who were already having seizures before the change in goals of care. The focus of seizure management in PC is on decreasing or alleviating the discomfort caused by seizures and may require medication dosages and routes that are usually not utilized for patients not under PC (109). As management for seizures ensue, concurrent management of other symptoms such as spasticity, increased secretions, pain and respiratory discomfort should continue (110,111). A proposed algorithm for management of patients with status epilepticus in the PC setting is shown in Figure 1. Because of the anticipated adverse effects of these medications, underlying principles of PS should be applied. Continued discussion with the family regarding goals of care should be done. The doctrine of double effect may serve as a guide to ethically manage status epilepticus in the PC setting. Further prospective studies should be done to guide management of status epilepticus in the setting of palliative level of care.
Tracheostomy placement
Tracheostomies are commonly done in several neurologic disorders. In the neuro-intensive care unit, this procedure is usually indicated for those patients who are unable to protect their airway due to the underlying brain injury and those who are unable to wean from the ventilator but require long-term mechanical ventilation (112). These patients include but are not limited to stroke (112), traumatic brain injuries (113), spinal cord injuries and neuromuscular disorders such as myasthenia gravis (114) or Guillain-Barré Syndrome (115). In the outpatient setting among patients with ALS, certain parameters such as a forced vital capacity of ≤50% trigger discussion regarding tracheostomy with invasive ventilation that has been shown to prolong survival but also are associated with more expense and an increased caregiver burden (116). There is no consensus regarding the timing of discussing tracheostomy placement in these disorders however various predictors have been published to guide physicians in discussing the likelihood of tracheostomy (112,115,117). Multiple factors eventually play a role in the final decision to proceed with a tracheostomy including the providers’ own views, the hospital’s practice, financial incentive, but most importantly the patients’ wishes expressed through the family (118). PC involvement is encouraged as early as possible to be part of the decision making even after tracheostomy. A retrospective study among TBI patients under PC showed that 65% of the patients under PC received a tracheostomy (119). This indicates that even with tracheostomy placement, PC involvement should continue. On the other hand, it has been shown that involvement of PC decreased performance of tracheostomy (118,120). The approach to the decision making should be balanced with what we know of the outcome of the underlying condition, patient comorbidities, what is uncertain regarding the prognosis, the risk and benefits of tracheostomy and how it may affect the patient and caregiver quality of life.
Feeding tube placement
Feeding tube placement among patients with neurological disorders are common most especially when dysphagia results from the disease. It encompass several tubes including an NGT, DHT which are usually not for long-term use and, PEG tubes or PEG-J tubes or surgical GT which may be used long-term and in the outpatient setting. For a patient to be able to have oral nutritional intake, they have to have intact level of consciousness, a learned ability to eat, adequate truncal and axial tone, proper coordination of neural mechanisms for swallowing, intact oral and nasal passage with a functional oro-digestive and respiratory tract. Neurologic disorders can potentially affect any of these key requirements for safe oral intake. Intracranial lesions can affect the level of consciousness to make it unable for patients to open their mouth, follow commands or use their basic known skills to eat, patients with spinal cord injuries in the acute phase may not be adequately be able to position themselves, patients with neuromuscular disorders have impaired tone that affect their oro-digestive tract. It has been shown that dysphagia is a predictor of transitioning to palliative level of care among stroke patients (121). The interaction between the ability to swallow, the decision to place a feeding tube and the decision to transition to palliative level of care has not been studied well. It has been shown that GT was an independent predictor of increased length of stay in a PC center (122). It has been shown as well that mortality after GT placement is high, although not directly related to GT placement, that PC involvement is strongly encouraged to discuss goals of care prior to GT placement (123). Decision making on PEG tube placement is not straightforward especially in patients with ALS where GT placement is recommended to stabilize weight and prolong survival (11) although the timing is still unclear. A recent report on GT placement for patients with prion disease was done and found that their tolerance of the procedure is similar to what has been published in literature for other conditions (124). It is important to be clear what is the effect or lack thereof of gastrostomy tube placement on the outcome of the disease based on evidence and to be transparent with potential complications. PC involvement should be continued even after GT placement because these patients will still require continued support through the course of their disease.
Disease specific considerations in PC management of patients with neurologic disorders
Several disease specific considerations are worth noting (Table 1). Patients with a focal neurologic lesion such as an ischemic stroke will have specific neurologic deficits that may pose as a challenge in management under PC. More importantly, there are stroke syndromes by which the level of consciousness and ability to communicate are directly affected. Several examples of these stroke syndromes are presented in Table 1 of note however; “stroke-like” syndromes with a similar presentation may be seen if the intracranial lesion affects a similar area compared to the vascular territory of the stroke. The patient in a vegetative and minimally conscious state is specifically shown due to the implications in PC management. It is important to emphasize that patients who are minimally conscious may have intact pain sensation (126). Note that several mechanisms of injury that lead to these states have implications on outcome which are beyond the scope of this review however as an example, a vegetative state due to TBI may have a better outcome than a patient with an anoxic brain injury (128). Although uncommon, CJD may pose as a challenge in PC management due to its fatal course, staff unfamiliarity with the disease and its transmission and the prominence of severe psychiatric and neurologic symptoms towards its terminal stages (130). ALS is one of the well-studied neurologic conditions in terms of PC management although similar to MS, PD and AD; the timing of PC involvement is still unclear. Unique to this group however is that it has been recommended that they undergo tracheostomy and gastrostomy for prolonged survival (11,116). This recommendation is not applicable to other chronic neurodegenerative or progressive disorders. Common to these conditions is cognitive impairment either as a part of the progression of disease (MS) or as a variant of the disease (ALS with Fronto-Temporal-Dementia or parkinsonian syndrome with Lewy Body Dementia) (131,134). These have implications on limitations of communication and expression of pain or discomfort. The challenge in managing AD, a chronic progressive cognitive disorder is that they usually have comorbidities that make them prone to multiple hospitalizations. Proactive discussion and early involvement of PC teams may play a role in improving their overall quality of life (138). Aside from the general challenges experienced in the PC management of patients with CNS malignancies, one specific consideration for these patients are steroid and AED tapering at the end-stage of their illness. If steroids are tapered, it may be helpful to increase the dose of the AED. If the patient develops seizures or progress to status epilepticus, they should be managed accordingly as part of PC (Figure 1). Neurology consult may be warranted.
Full table
Conclusions
There is an overall increase in awareness and research on the role of PC in the management of patients with neurologic disorders. There are however unique challenges and ethical issues that are important to consider in the management of these patients. Further prospective studies are warranted to examine the effect of PC management on patient and family outcome as well as studies to systematically analyze other means to improve PC utilization among patients with neurologic conditions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dying in America: improving quality and honoring individual preferences near the end of life. Mil Med 2015;180:365-7. [Crossref] [PubMed]
- World Health Organization. WHO definition of palliative care. World Health Organization 2017; Available online: http://www.who.int/cancer/palliative/definition/en/
- Dahlin C. National consensus project for quality palliative care task force. clinical practice guidelines for quality palliative care. Pittsburgh, PA: National Consensus Project for Quality Palliative Care, 2013.
- Braun LT, Grady KL, Kutner JS, et al. Palliative care and cardiovascular disease and stroke: a policy statement from the American heart association/American stroke association. Circulation 2016;134:e198-225. [Crossref] [PubMed]
- Position of the American academy of neurology on certain aspects of the care and management of the persistent vegetative state patient. Adopted by the executive board, American academy of neurology, April 21, 1988, Cincinnati, Ohio. Neurology 1989;39:125-6. [PubMed]
- Dallara A, Tolchin DW. Emerging subspecialties in neurology: palliative care. Neurology 2014;82:640-2. [Crossref] [PubMed]
- Bernat JL, Goldstein ML, Viste KM Jr. The neurologist and the dying patient. Neurology 1996;46:598-9. [Crossref] [PubMed]
- Palliative care in neurology. The American academy of neurology ethics and humanities subcommittee. Neurology 1996;46:870-2. [PubMed]
- Oliver DJ, Borasio GD, Caraceni A, et al. A consensus review on the development of palliative care for patients with chronic and progressive neurological disease. Eur J Neurol 2016;23:30-8. [Crossref] [PubMed]
- Bauchamp TL, Childress JF, Oxford University Press. Principles of biomedical ethics. New York, Oxford: Oxford University Press, 2013.
- Miller RG, Rosenberg JA, Gelinas DF, et al. Practice parameter: the care of the patient with amyotrophic lateral sclerosis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology: ALS Practice Parameters Task Force. Neurology 1999;52:1311-23. [Crossref] [PubMed]
- Holloway RG, Arnold RM, Creutzfeldt CJ, et al. Palliative and end-of-life care in stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1887-916. [Crossref] [PubMed]
- Frontera JA, Curtis JR, Nelson JE, et al. Integrating palliative care into the care of neurocritically Ill patients: a report from the improving palliative care in the icu project advisory board and the center to advance palliative care. Crit Care Med 2015;43:1964-77. [Crossref] [PubMed]
- Meyers FJ, Linder J. Simultaneous care: disease treatment and palliative care throughout illness. J Clin Oncol 2003;21:1412-5. [Crossref] [PubMed]
- Meyers FJ, Linder J, Beckett L, et al. Simultaneous care: a model approach to the perceived conflict between investigational therapy and palliative care. J Pain Symptom Manage 2004;28:548-56. [Crossref] [PubMed]
- Kelley AS, Meier DE. Palliative care--a shifting paradigm. N Engl J Med 2010;363:781-2. [Crossref] [PubMed]
- Provinciali L, Carlini G, Tarquini D, et al. Need for palliative care for neurological diseases. Neurol Sci 2016;37:1581-7. [Crossref] [PubMed]
- Stoff BK, Seidler A, Kinlaw K. A patient with dementia: decision-making capacity and surrogate decision makers. J Am Acad Dermatol 2012;67:745-7. [Crossref] [PubMed]
- Khin Khin E, Minor D, Holloway A, et al. Decisional capacity in amyotrophic lateral sclerosis. J Am Acad Psychiatry Law 2015;43:210-7. [PubMed]
- Bernat JL. Ethical issues in the treatment of severe brain injury: the impact of new technologies. Ann N Y Acad Sci 2009;1157:117-30. [Crossref] [PubMed]
- Chahine LM, Malik B, Davis M. Palliative care needs of patients with neurologic or neurosurgical conditions. Eur J Neurol 2008;15:1265-72. [Crossref] [PubMed]
- Sabatino C. Myths and facts about health care advance directives. BiFocal 2015;37:6-9.
- Cai X, Robinson J, Muehlschlegel S, et al. Patient preferences and surrogate decision making in neuroscience intensive care units. Neurocrit Care 2015;23:131-41. [Crossref] [PubMed]
- Wynn S. Decisions by surrogates: an overview of surrogate consent laws in the United States. BiFocal 2014;36:10-3.
- Karp N, Wood E. Incapacitated and alone: healthcare decision making for unbefriended older people. Human Rights 2004;31:20-3.
- Pope TM, Sellers T. Legal briefing: the unbefriended: making healthcare decisions for patients without surrogates (Part 2). J Clin Ethics 2012;23:177-92. [PubMed]
- Making treatment decisions for incapacitated older adults without advance directives. AGS Ethics Committee. American Geriatrics Society. J Am Geriatr Soc 1996;44:986-7. [Crossref] [PubMed]
- White DB, Curtis JR, Wolf LE, et al. Life support for patients without a surrogate decision maker: who decides? Ann Intern Med 2007;147:34-40. [Crossref] [PubMed]
- Sequeira AL, Lewis A. Ethical and legal considerations in the management of an unbefriended patient in a vegetative state. Neurocrit Care 2017;27:173-9. [Crossref] [PubMed]
- Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med 2012;366:780-1. [Crossref] [PubMed]
- Berger JT, DeRenzo EG, Schwartz J. Surrogate decision making: reconciling ethical theory and clinical practice. Ann Intern Med 2008;149:48-53. [Crossref] [PubMed]
- Johansson M, Brostrom L. Empirical fallacies in the debate on substituted judgment. Health Care Anal 2014;22:73-81. [Crossref] [PubMed]
- Torke AM, Alexander GC, Lantos J. Substituted judgment: the limitations of autonomy in surrogate decision making. J Gen Intern Med 2008;23:1514-7. [Crossref] [PubMed]
- Cronberg T, Kuiper M. Withdrawal of life-sustaining therapy after cardiac arrest. Semin Neurol 2017;37:81-7. [Crossref] [PubMed]
- Bouwes A, Binnekade JM, Kuiper MA, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol 2012;71:206-12. [Crossref] [PubMed]
- Fugate JE, Wijdicks EF, White RD, et al. Does therapeutic hypothermia affect time to awakening in cardiac arrest survivors? Neurology 2011;77:1346-50. [Crossref] [PubMed]
- Mulder M, Gibbs HG, Smith SW, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia*. Crit Care Med 2014;42:2493-9. [Crossref] [PubMed]
- Nolan JP, Soar J, Cariou A, et al. European resuscitation council and European society of intensive care medicine 2015 guidelines for post-resuscitation care. Intensive Care Med 2015;41:2039-56. [Crossref] [PubMed]
- Weimer JM, Nowacki AS, Frontera JA. Withdrawal of life-sustaining therapy in patients with intracranial hemorrhage: self-fulfilling prophecy or accurate prediction of outcome? Crit Care Med 2016;44:1161-72. [Crossref] [PubMed]
- Rocker G, Cook D, Sjokvist P, et al. Clinician predictions of intensive care unit mortality. Crit Care Med 2004;32:1149-54. [Crossref] [PubMed]
- Heeley E, Anderson CS, Woodward M, et al. Poor utility of grading scales in acute intracerebral hemorrhage: results from the INTERACT2 trial. Int J Stroke 2015;10:1101-7. [Crossref] [PubMed]
- Xu J, Tao Y, Xie X, et al. A Comparison of mortality prognostic scores in ischemic stroke patients. J Stroke Cerebrovasc Dis 2016;25:241-7. [Crossref] [PubMed]
- Naval NS, Kowalski RG, Chang TR, et al. The SAH score: a comprehensive communication tool. J Stroke Cerebrovasc Dis 2014;23:902-9. [Crossref] [PubMed]
- Hansen BM, Morgan TC, Betz JF, et al. Intraventricular extension of supratentorial intracerebral hemorrhage: the modified graeb scale improves outcome prediction in lund stroke register. Neuroepidemiology 2016;46:43-50. [Crossref] [PubMed]
- Carter EL, Hutchinson PJ, Kolias AG, et al. Predicting the outcome for individual patients with traumatic brain injury: a case-based review. Br J Neurosurg 2016;30:227-32. [Crossref] [PubMed]
- Schuster C, Hardiman O, Bede P. Survival prediction in Amyotrophic lateral sclerosis based on MRI measures and clinical characteristics. BMC Neurol 2017;17:73. [Crossref] [PubMed]
- Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 1: patients not treated with therapeutic hypothermia. Resuscitation 2013;84:1310-23. [Crossref] [PubMed]
- Turgeon AF, Lauzier F, Simard JF, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ 2011;183:1581-8. [Crossref] [PubMed]
- Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology 2007;68:1651-7. [Crossref] [PubMed]
- Quill TE. Principle of double effect and end-of-life pain management: additional myths and a limited role. J Palliat Med 1998;1:333-6. [Crossref] [PubMed]
- Lindblad A, Lynöe N, Juth N. End-of-life decisions and the reinvented Rule of Double Effect: a critical analysis. Bioethics. 2014;28:368-77. [Crossref] [PubMed]
- Alpers A. Criminal act or palliative care? Prosecutions involving the care of the dying. J Law Med Ethics 1998;26:308-31, 262.
- McCormick R. The principle of double effect. In: How brave a new world? Dilemmas in bioethics. Washington, DC: Georgetown University Press, 1981.
- Thorns A, Sykes N. Opioid use in last week of life and implications for end-of-life decision-making. Lancet 2000;356:398-9. [Crossref] [PubMed]
- Kendall CE. At the coalface: medical ethics in practice. A double dose of double effect. J Med Ethics 2000;26:204-5. [Crossref] [PubMed]
- Bonito V, Caraceni A, Borghi L, et al. The clinical and ethical appropriateness of sedation in palliative neurological treatments. Neurol Sci 2005;26:370-85. [Crossref] [PubMed]
- Maltoni M, Pittureri C, Scarpi E, et al. Palliative sedation therapy does not hasten death: results from a prospective multicenter study. Ann Oncol 2009;20:1163-9. [Crossref] [PubMed]
- Ventafridda V, Ripamonti C, De Conno F, et al. Symptom prevalence and control during cancer patients' last days of life. J Palliat Care. 1990;6:7-11. [PubMed]
- Sykes N, Thorns A. The use of opioids and sedatives at the end of life. Lancet Oncol 2003;4:312-8. [Crossref] [PubMed]
- Stone P, Phillips C, Spruyt O, et al. A comparison of the use of sedatives in a hospital support team and in a hospice. Palliat Med 1997;11:140-4. [Crossref] [PubMed]
- Morita T, Tsunoda J, Inoue S, et al. Effects of high dose opioids and sedatives on survival in terminally ill cancer patients. J Pain Symptom Manage 2001;21:282-9. [Crossref] [PubMed]
- Chiu TY, Hu WY, Lue BH, et al. Sedation for refractory symptoms of terminal cancer patients in Taiwan. J Pain Symptom Manage 2001;21:467-72. [Crossref] [PubMed]
- Sykes N, Thorns A. Sedative use in the last week of life and the implications for end-of-life decision making. Arch Intern Med 2003;163:341-4. [Crossref] [PubMed]
- Rady MY, Verheijde JL. Sedation for the imminently dying: survey results from the AAN Ethics Section. Neurology 2010;75:1753. [Crossref] [PubMed]
- Fainsinger RL, Landman W, Hoskings M, et al. Sedation for uncontrolled symptoms in a South African hospice. J Pain Symptom Manage 1998;16:145-52. [Crossref] [PubMed]
- Fainsinger RL, Waller A, Bercovici M, et al. A multicentre international study of sedation for uncontrolled symptoms in terminally ill patients. Palliat Med 2000;14:257-65. [Crossref] [PubMed]
- Muller-Busch HC, Andres I, Jehser T. Sedation in palliative care - a critical analysis of 7 years experience. BMC Palliat Care 2003;2:2. [Crossref] [PubMed]
- Porta J. Palliative sedation: clinical aspects. In: Between technology and humanity. Leuven, Belgium: Leuven University Press, 2002.
- Voltz R, Borasio GD. Palliative therapy in the terminal stage of neurological disease. J Neurol 1997;244:S2-10. [Crossref] [PubMed]
- deBacker J, Hart N, Fan E. Neuromuscular Blockade in the 21st Century Management of the Critically Ill Patient. Chest 2017;151:697-706. [Crossref] [PubMed]
- Pigazzi A, Manfredi PL. Commentary: the role of neuromuscular blockade in palliative medicine. J Pain Symptom Manage 2000;19:155-6. [Crossref]
- Perkin RM, Resnik DB. The agony of agonal respiration: is the last gasp necessary? J Med Ethics 2002;28:164-9. [Crossref] [PubMed]
- Resnik MD. Choices: an introduction to decision theory. Minneapolis, Minnesota: University of Minnesota Press, 1987:26-7.
- Druml C, Ballmer PE, Druml W, et al. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin Nutr 2016;35:545-56. [Crossref] [PubMed]
- Smith L, Ferguson R. Artificial nutrition and hydration in people with late-stage dementia. Home Healthc Now 2017;35:321-5. [Crossref] [PubMed]
- Hospice and palliative nurses association position statement: artificial nutrition and hydration in advanced illness. Pittsburgh, PA: Hospice and Palliative Nurses Association, 2011.
- American geriatrics society ethics committee and clinical practice and models of care committee. American geriatrics society feeding tubes in advanced dementia position statement. J Am Geriatr Soc 2014;62:1590-3. [Crossref]
- Ciocon JO, Silverstone FA, Graver LM, et al. Tube feedings in elderly patients. Indications, benefits, and complications. Arch Intern Med 1988;148:429-33. [Crossref] [PubMed]
- Finucane TE, Christmas C, Travis K. Tube feeding in patients with advanced dementia: a review of the evidence. JAMA 1999;282:1365-70. [Crossref] [PubMed]
- Odom SR, Barone JE, Docimo S, et al. Emergency department visits by demented patients with malfunctioning feeding tubes. Surg Endosc 2003;17:651-3. [Crossref] [PubMed]
- Lembeck ME, Pameijer CR, Westcott AM. The role of intravenous fluids and enteral or parenteral nutrition in patients with life-limiting illness. Med Clin North Am 2016;100:1131-41. [Crossref] [PubMed]
- Hanson LC, Ersek M, Gilliam R, et al. Oral feeding options for people with dementia: a systematic review. J Am Geriatr Soc 2011;59:463-72. [Crossref] [PubMed]
- Bruera E, Franco JJ, Maltoni M, et al. Changing pattern of agitated impaired mental status in patients with advanced cancer: association with cognitive monitoring, hydration, and opioid rotation. J Pain Symptom Manage 1995;10:287-91. [Crossref] [PubMed]
- MacDonald SM, Fainsinger RL. Symptom control: the problem areas. Palliat Med 1994;8:167-8. [Crossref] [PubMed]
- Blinderman CD, Billings JA. Comfort care for patients dying in the hospital. N Engl J Med 2015;373:2549-61. [Crossref] [PubMed]
- Larriviere D, Bonnie RJ. Terminating artificial nutrition and hydration in persistent vegetative state patients: current and proposed state laws. Neurology 2006;66:1624-8. [Crossref] [PubMed]
- Seliger SL, Gillen DL, Longstreth WT Jr, et al. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int 2003;64:603-9. [Crossref] [PubMed]
- Masson P, Kelly PJ, Craig JC, et al. Risk of stroke in patients with ESRD. Clin J Am Soc Nephrol 2015;10:1585-92. [Crossref] [PubMed]
- Iseki K, Kinjo K, Kimura Y, et al. Evidence for high risk of cerebral hemorrhage in chronic dialysis patients. Kidney Int 1993;44:1086-90. [Crossref] [PubMed]
- Wang IK, Lin CL, Wu YY, et al. Subdural hematoma in patients with end-stage renal disease receiving hemodialysis. Eur J Neurol 2014;21:894-900. [Crossref] [PubMed]
- Di Lullo L, Rivera R, Barbera V, et al. Sudden cardiac death and chronic kidney disease: From pathophysiology to treatment strategies. Int J Cardiol 2016;217:16-27. [Crossref] [PubMed]
- Association RP. Shared decision-making in the appropriate initiation and withdrawal from dialysis. Rockville, Maryland; 2010.
- Wang WY, Sang WW, Jin D, et al. The prognostic value of the iscore, the plan score, and the ASTRAL score in acute ischemic stroke. J Stroke Cerebrovasc Dis 2017;26:1233-8. [Crossref] [PubMed]
- Hemphill JC 3rd, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891-7. [Crossref] [PubMed]
- Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008;39:2304-9. [Crossref] [PubMed]
- Lunetta C, Lizio A, Melazzini MG, et al. Amyotrophic lateral sclerosis survival score (ALS-SS): A simple scoring system for early prediction of patient survival. Amyotroph Lateral Scler Frontotemporal Degener 2015;17:93-100. [Crossref] [PubMed]
- Quill TE, Holloway R. Time-limited trials near the end of life. JAMA 2011;306:1483-4. [Crossref] [PubMed]
- Patel SS, Holley JL. Withholding and withdrawing dialysis in the intensive care unit: benefits derived from consulting the renal physicians association/American society of nephrology clinical practice guideline, shared decision-making in the appropriate initiation of and withdrawal from dialysis. Clin J Am Soc Nephrol 2008;3:587-93. [Crossref] [PubMed]
- Skold A, Lesandrini J, Gorbatkin S. Ethics and health policy of dialyzing a patient in a persistent vegetative state. Clin J Am Soc Nephrol 2014;9:366-70. [Crossref] [PubMed]
- Ford J, Sears S, Ramza B, et al. The registry evaluating functional outcomes of resynchronization management (REFORM): quality of life and psychological functioning in patients receiving cardiac resynchronization therapy. J Cardiovasc Electrophysiol 2014;25:43-51. [Crossref] [PubMed]
- Ayach B, Malik A, Seifer C, et al. End of life decisions in heart failure: to turn off the intracardiac device or not? Curr Opin Cardiol 2017;32:224-8. [PubMed]
- Frontera JA. How I manage the adult potential organ donor: donation after cardiac death (part 2). Neurocrit Care 2010;12:111-6. [Crossref] [PubMed]
- Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia 2005;46:470-2. [Crossref] [PubMed]
- Hantus S. Epilepsy emergencies. Continuum (Minneap Minn) 2016;22:173-90. [Crossref] [PubMed]
- Samala RV, Parala-Metz A, Davis MP. Nonconvulsive status epilepticus in a palliative care unit: when delirium is a seizure. Am J Hosp Palliat Care 2015;32:243-7. [Crossref] [PubMed]
- Golf M, Paice JA, Feulner E, et al. Refractory status epilepticus. J Palliat Med 2004;7:85-8. [Crossref] [PubMed]
- Claassen J, Silbergleit R, Weingart SD, et al. Emergency neurological life support: status epilepticus. Neurocrit Care. 2012;17:S73-8. [Crossref] [PubMed]
- Droney J, Hall E. Status epilepticus in a hospice inpatient setting. J Pain Symptom Manage 2008;36:97-105. [Crossref] [PubMed]
- Sizoo EM, Koekkoek JA, Postma TJ, et al. Seizures in patients with high-grade glioma: a serious challenge in the end-of-life phase. BMJ Support Palliat Care 2014;4:77-80. [Crossref] [PubMed]
- Cancelli F, Dubra A, Zulian GB. Palliative sedation for status epilepticus in a patient with progressive multifocal leukoencephalopathy. J Pain Palliat Care Pharmacother 2014;28:382-3. [Crossref] [PubMed]
- Dulin JD, Noreika DM, Coyne PJ. Management of refractory status epilepticus in an actively dying patient. J Pain Palliat Care Pharmacother 2014;28:243-50. [Crossref] [PubMed]
- Schönenberger S, Al-Suwaidan F, Kieser M, et al. The SETscore to predict tracheostomy need in cerebrovascular neurocritical care patients. Neurocrit Care 2016;25:94-104. [Crossref] [PubMed]
- Shibahashi K, Sugiyama K, Houda H, et al. The effect of tracheostomy performed within 72 h after traumatic brain injury. Br J Neurosurg 2017;31:564-8. [PubMed]
- Rabinstein AA. Noninvasive ventilation for neuromuscular respiratory failure: when to use and when to avoid. Curr Opin Crit Care 2016;22:94-9. [PubMed]
- Walgaard C, Lingsma HF, van Doorn PA, et al. Tracheostomy or not: prediction of prolonged mechanical ventilation in Guillain-Barré syndrome. Neurocrit Care 2017;26:6-13. [Crossref] [PubMed]
- Danel-Brunaud V, Touzet L, Chevalier L, et al. Ethical considerations and palliative care in patients with amyotrophic lateral sclerosis: a review. Rev Neurol (Paris) 2017;173:300-7. [Crossref] [PubMed]
- Szeder V, Ortega-Gutierrez S, Ziai W, et al. The TRACH score: clinical and radiological predictors of tracheostomy in supratentorial spontaneous intracerebral hemorrhage. Neurocrit Care 2010;13:40-6. [Crossref] [PubMed]
- Holloway RG, Quill TE. Treatment decisions after brain injury--tensions among quality, preference, and cost. N Engl J Med 2010;362:1757-9. [Crossref] [PubMed]
- Kahveci K, Dincer M, Doger C, et al. Traumatic brain injury and palliative care: a retrospective analysis of 49 patients receiving palliative care during 2013-2016 in Turkey. Neural Regen Res 2017;12:77-83. [Crossref] [PubMed]
- Pan CX, Gutierrez C, Maw MM, et al. Impact of a Palliative Care Program on Tracheostomy Utilization in a Community Hospital. J Palliat Med 2015;18:1070-3. [Crossref] [PubMed]
- San Luis CO, Staff I, Fortunato GJ, et al. Dysphagia as a predictor of outcome and transition to palliative care among middle cerebral artery ischemic stroke patients. BMC Palliat Care 2013;12:21. [Crossref] [PubMed]
- Dincer M, Kahveci K, Doger C. An examination of factors affecting the length of stay in a palliative care center. J Palliat Med 2018;21:11-5. [Crossref] [PubMed]
- Meisel K, Arnold RM, Stijacic Cenzer I, et al. Survival, functional status, and eating ability after percutaneous endoscopic gastrostomy tube placement for acute stroke. J Am Geriatr Soc 2017;65:1848-52. [Crossref] [PubMed]
- Iwasaki Y, Mori K, Ito M, et al. Gastrostomy in patients with prion disease. Prion 2017;11:186-94. [Crossref] [PubMed]
- Eapen BC, Georgekutty J, Subbarao B, et al. Disorders of Consciousness. Phys Med Rehabil Clin N Am 2017;28:245-58. [Crossref] [PubMed]
- Fins JJ, Master MG, Gerber LM, et al. The minimally conscious state: a diagnosis in search of an epidemiology. Arch Neurol 2007;64:1400-5. [Crossref] [PubMed]
- Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002;58:349-53. [Crossref] [PubMed]
- Posner JB, Saper CB, Schiff N, et al. Plum and posner's diagnosis of stupor and coma. 4th ed. S. G, editor. New York: Oxford University Press, 2007.
- Chen C, Dong XP. Epidemiological characteristics of human prion diseases. Infect Dis Poverty 2016;5:47. [Crossref] [PubMed]
- de Vries K, Sque M, Bryan K, et al. Variant Creutzfeldt-Jakob disease: need for mental health and palliative care team collaboration. Int J Palliat Nurs 2003;9:512-20. [Crossref] [PubMed]
- Couratier P, Corcia P, Lautrette G, et al. ALS and frontotemporal dementia belong to a common disease spectrum. Rev Neurol (Paris) 2017;173:273-9. [Crossref] [PubMed]
- Elman LB, Houghton DJ, Wu GF, et al. Palliative care in amyotrophic lateral sclerosis, Parkinson's disease, and multiple sclerosis. J Palliat Med 2007;10:433-57. [Crossref] [PubMed]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-52. [Crossref] [PubMed]
- Higginson IJ, McCrone P, Hart SR, et al. Is short-term palliative care cost-effective in multiple sclerosis? A randomized phase II trial. J Pain Symptom Manage 2009;38:816-26. [Crossref] [PubMed]
- Scharre DW, Chang SI, Nagaraja HN, et al. Paired studies comparing clinical profiles of lewy body dementia with alzheimer's and parkinson's diseases. J Alzheimers Dis 2016;54:995-1004. [Crossref] [PubMed]
- Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry 1996;60:615-20. [Crossref] [PubMed]
- Wenning GK, Litvan I, Jankovic J, et al. Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry 1998;64:184-9. [Crossref] [PubMed]
- Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009;361:1529-38. [Crossref] [PubMed]
- Tsao MN, Rades D, Wirth A, et al. International practice survey on the management of brain metastases: third international consensus workshop on palliative radiotherapy and symptom control. Clin Oncol (R Coll Radiol) 2012;24:e81-92. [Crossref] [PubMed]
- Modi A, Vohra HA, Weeden DF. Does surgery for primary non-small cell lung cancer and cerebral metastasis have any impact on survival? Interact Cardiovasc Thorac Surg 2009;8:467-73. [Crossref] [PubMed]
- Jeon YS, Koh YC, Song SW, et al. Palliative resection of metastatic brain tumors previously treated by stereotactic radiosurgery. Brain Tumor Res Treat 2016;4:116-23. [Crossref] [PubMed]