Supportive & palliative interventions in motor neurone disease: what we know from current literature?
Introduction
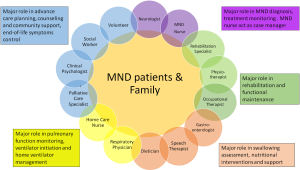
The treatment of motor neurone disease (MND) incorporates the management of complex medical issues, psychosocial considerations as well as functional disabilities. A coordinated team of medical, nursing and allied health professionals constitutes a multidisciplinary care (MDC) model that results in improved care and quality of life (QOL), reduction in the length and frequency of hospitalization and prolongation of survival in MND patients (1,2). The multidisciplinary clinic typically involves a neurologist, MND nurse, physiotherapist, occupational and speech therapists.
Methods
Evidence for this review was obtained from a search of the Cochrane data base, PubMed, guidelines of National Institute for Clinical Excellence (NICE), American Academy of Neurology (AAN), and European Federation of Neurological Societies (EFNS); and peer-reviewed journal articles. MND diagnosis is based on the El Escorial diagnostic criteria (3).
Objectives
This review aims to objectively evaluate the role of the supportive interventions available to patients with MND, the evidence basis for intervention modalities, and highlight areas for future research. The benefit(s) of intervention measures are assessed on their impact on outcomes such as survival, QOL, reduced hospitalization, improved disability, and cost effectiveness.
This integrative review includes articles from the earlier review as well as articles that were found through database searches on articles published from 2003 through March 2015. In addition, we reviewed bibliographies of selected articles for potentially relevant articles, and other articles recommended by experts in the field were included in this review. Search terms for the review included supportive care, multidisciplinary approach, symptoms activities, activities of daily living, assistive devices, assistive equipment, caregiver support, cognition, disease management, education, emotional regulation, exercise, extended care, fall prevention, fatigue, health maintenance, instrumental activities of daily living, intervention, leisure, mobility, mobility equipment, neurorehabilitation, occupational therapy, QOL, rehabilitation, self-management, sleep, social engagement, walkers, wheelchairs, and work.
Results
MDC
MDC approach is the mainstay for the management of patients with chronic neurological conditions such as multiple sclerosis (4), stroke (5), acquired brain injury (6), and MND. MDC is defined as any care delivered by two or more disciplines (7), involving a neurologist and other allied disciplines such as MND nurse, chest physician, and occupational therapist. Other personnel needed as part of the MDC team for MND care includes occupational therapists, physiotherapists, social workers, palliative care (PC) physician, speech and language therapist, and religious leaders. Individual role of various MDC team members are summarized in Figure 1. Care is administered 24 hours daily in a hospital or on outpatient basis or in the patients’ home or community, but effort must be effectively coordinated to avoid overlapping or missing care due to the large number of care providers involved in the management of the patient and their family. MDC is important in enabling care specialist to undertake proper assessment of patients and addressing the concerns of patients and family (8,9).
MDC is reported to prolong survival from 7 to 24 months, possibly further enhanced in patients with bulbar disease (10), while reducing the risk of death by 45% at 5 years. Such a model may also provide support for the treating clinicians coordinating the complex care requirements. However, challenges still exist. Patients may describe an uneven deterioration as their disease progresses, despite the fact that the progression profile of MND tends to remain relatively linear. To minimize and more optimally address these changes in care need, an anticipatory model with a focus on proactive intervention represents the ideal standard. Overall, the survival benefits achieved through MDC outweigh that of other interventions, including pharmacological treatment. As such, this framework should be implemented as the standard of care for all patients in the management of this rapidly progressive neurological disorder.
A study (10) retrospectively reviewed hospital notes of 162 patients seen between 1998 and 2002 in general neurology clinic (GNC) and 255 others managed under MDC care between 2006 and 2010 in a tertiary hospital. Median survival from diagnosis was 19 months for MDC and 11 months for GNC (hazard ratio 0.51, 95% confidence interval, 0.41–0.64). They also analysed the relationship between MDC and survival independent of riluzole, non-invasive ventilation (NIV), and percutaneous endoscopic gastrostomy (PEG) use. Although this study selected patients from multiple neurologists in the region, a rigorous methodology was used to ensure proper patient selection and matching. Other factors that are being suggested as contributing to an improved outcome in MDC setting include better symptomatic support, access to aids, and prompt treatment of respiratory challenges (2,11). Both AAN and EFNS recommend MDC care setting for patients with MND, with the current EFNS guidelines recognizing the benefit of MDC approach in improving survival, reducing medical complications, and improving the QOL of patients and their caregivers (12,13).
Symptomatic management
Respiratory management
Respiratory impairment is the leading cause of death in MND. Symptoms of respiratory dysfunction in MND patients include sleep disturbance, excessive daytime somnolence, morning headaches and cognitive changes. Almost all MND patients will develop respiratory problems during the course of their disease. In a small percentage of MND patients, respiratory failure may present as the primary symptom at onset, whereas more commonly it develops later in the disease. It is the cause of death in most MND patients (14), representing an important negative survival factor. Ideally, evaluation of respiratory function should be undertaken at each clinic visit. Forced vital capacity (FVC), taken either supine or erect, is the most commonly used measurement tool of respiratory function. Such measures are well recognized predictors of survival (15), with supine FVC a more accurate marker of diaphragmatic weakness (14). However, FVC may not be sensitive for the detection of early respiratory failure and can be technically difficult to perform in patients with severe bulbar weakness (16). Other respiratory measures include maximal inspiratory pressure (MIP), maximal expiratory pressure, sniff nasal inspiratory pressure (SNIP) and, less commonly, formal assessment of arterial blood gases. Interpretation of the trends of these measures over time, combined with the clinical picture, determines appropriate respiratory management. Significantly, the advent of NIV has provided clear benefit in terms of improving symptoms, QOL and prolonging survival by up to 7 months, making NIV a central armamentarium of respiratory management in MND patients (17-19). Bilevel ventilation devices (Bi-PAP) are most commonly used as initial therapy. There are no established evidence-based guidelines regarding optimal timing for initiation of NIV. Current recommendations for considering a trial of NIV include PCO2 more than 45 mmHg, MIP less than 60% predicted, FVC less than 50% predicted, oxygen saturation less than 88% for at least 5 continuous minutes during sleep or SNIP less than 40% predicted. Some studies suggest that an early introduction of NIV may increase survival (20,21), reduce respiratory-related energy expenditure (22) and improve adherence to therapy and QOL (23). Clearly, formal studies are needed to better define the precise timing of this intervention. Once initiated, poor optimization of NIV represents an independent risk factor for mortality (24). However, there are no randomized control trial data available that have compared the specific parameters of bilevel modes of ventilation across various patient cohorts. Different strategies have been used to optimize patient comfort, including adjusting the type of mask and fittings, providing humidified air and testing different Bi-PAP pressure settings. Despite these measures, up to 30% of MND patients cannot tolerate therapy due to secondary effects of anxiety, emotional lability from pseudobulbar palsy, excessive salivation, claustrophobia and nasal bridge soreness (25). Importantly, the key factors that affect overall compliance with this treatment are the presence of cognitive impairment (26) or bulbar dysfunction (27), with bulbar onset patients six times less likely to tolerate NIV than those with limb onset disease (28). Invasive ventilatory support has been explored as an option for MND patients not able to utilize NIV. In patients with severe bulbar dysfunction and in those whose respiratory function has deteriorated despite the use of NIV, tracheostomy remains a consideration. Although this provides adequate respiratory support and simplifies the suction of secretions, tracheostomy is associated with an increased risk of lung infection, site infection, bleeding and tracheoesophageal fistula formation (29). It has been suggested that the majority of the complications may relate to the use of cuffed tracheostomy tubes, with one study showing that 48 of 52 cuffed tracheostomy tube users died from tube-related complications (30). In addition, Sancho et al. (31) demonstrated prolonged survival in patients with the use of intermittent positive pressure ventilatory support using uncuffed tracheostomy tubes. However, the use of the uncuffed tubes is transient in current clinical practice, often only employed until excessive air-leaks and/or hypoventilation demand a cuffed tracheostomy tube. Another invasive option that has been actively explored recently is electrical stimulation of the diaphragm, referred to as diaphragmatic pacing. This intervention was originally developed to assist patients with spinal cord injuries. A pilot study conducted in 16 MND patients showed benefits in terms of a reduction in FVC decline, quantitative improvement of diaphragmatic movement and the delayed need for respiratory support (32). However, a more recent randomized controlled trial contradicted these findings, suggesting that diaphragm pacing may conversely reduce survival, possibly by affecting vulnerable motor neurones or causing excessive muscle fatigue (33). Ultimately, however, performing any invasive treatment in a progressive and incurable disease remains an ethically challenging concept. Future efforts must be focused on determining more accurate testing for early respiratory failure, optimal time to initiate NIV and the comfortable adaptation of NIV devices for patients with bulbar weakness.
Respiration can also be assisted using an electrical stimulation of the diaphragm to produce contractions (diaphragmatic pacing). It is a procedure originally meant for patients with cervical spine injury (34), but still experimental in MND. Four electrodes are placed on the motor roots of the phrenic nerves on the abdominal surface of the diaphragm. Thus, it is only effective if the diaphragm still retains some innervation (33). The diaphragmatic pacing in patients with respiratory muscle weakness due to motor neurone disease study (DIPALS) is an ongoing randomized controlled trial (RCT) assessing the efficacy of diaphragmatic pacing among MND patients in some UK hospitals.
Apart from hypoventilation, respiratory weakness also impairs cough (35). Insufficient cough results in recurrent chest infection, which is the leading cause of hospitalization in MND (36). The strength of the patients’ cough is assessed with a peak flow meter and reported as suboptimal if the peak cough-flow (PCF) is less than 270/min (37). Cough can be augmented using intensive physiotherapy and manoeuvres like tussive squeeze and mechanical in-exsufflator (MI-E). Evidence for MI-E is weak, but it has been suggested that it could be effective in MND patients for cough management (38,39).
Nutritional management
Metabolic changes described in MND patients include fluctuations in weight, cholesterol and insulin resistance and are linked to disease progression and prognosis (40). Clinically asymptomatic thiamine deficiency has been reported in 28% of MND patients, with Wernicke encephalopathy found in approximately 10% of cases (41). In addition, elevated serum lipids have recently been identified as a potential positive factor for survival (42). Weight loss is associated with a more rapid disease progression and is a negative survival factor (43,44). The causes of malnutrition appear multi-factorial and can result from swallowing dysfunction, feeding difficulties due to loss of limb strength and dexterity, neuropsychiatric issues and the development of a hypermetabolic state (45,46). Weight must be regularly monitored, and weight loss and dysphagia ideally managed by an experienced speech pathologist, with video-fluoroscopy review and guidance in food consistency and safe swallowing techniques (such as chin tuck and upright position). Occupational therapists can assist with functional aids for feeding difficulties including provision of specialized cutlery, with support from carers and family members for additional assistance at meal times. Dieticians can assist with nutritional supplementation, including thiamine replacement (41). The benefits of various diets such as high protein, high fat, high carbohydrate and high calorie intake on disease progression have been trialled, with inconsistent results (47). With disease progression, malnutrition may inevitably occur, requiring more invasive nutritional intervention. The option for enteral feeding should be discussed early in the course of the disease and considered when 10% or more of baseline weight has been lost (44). If appropriate, nutritional interventions can be commenced via nasogastric tube or via gastrostomy insertion, either through percutaneous endoscopic gastrostomy (PEG), percutaneous radiological insertion of gastrostomy (RIG) or per-oral image-guided gastrostomy (PIG) (48,49).
Currently, there is no comparative data regarding differences in median survival or patient tolerance between different feeding mode, and the choice is often guided by clinical practice and experience. A nasogastric feeding tube (NG-tube) is passed through the nares, down the oesophagus and into the stomach, primary advantage of the NG-tube is that it is temporary and relatively non-invasive to place, meaning it can be removed or replaced at any time without surgery. NG-tubes can have complications, particularly related to accidental removal of the tube and nasal irritation. Besides, it may create negative cosmetic impact and require frequent changes. PEG is the most commonly used method, but can be hazardous in patients with concurrent respiratory muscle weak- ness and moderate-to-severe respiratory failure (mainly due to the risk of aspiration). This must therefore be discussed before either respiratory failure or severe dysphagia occurs. RIG has a higher success rate and is better tolerated, and as minimal or local sedation is required it can be considered in patients with impaired respiratory function (even when FVC is less than 50%) (46). Similarly, the newer fluoroscopic PIG technique uses minimal sedation and has reported lower complication rates, but is not yet as widely practised (50). If gastrostomy is contraindicated, home parenteral nutrition is occasionally considered for longer term nutritional support, with recent evidence showing improvement in nutritional status for these patients (51). However, this is inconsistently and uncommonly used in clinical practice. The complications of gastrostomy insertion can be related to the surgery (e.g., gastric haemorrhage, localized infection, peritonitis or failure of PEG placement), direct gastrointestinal changes (e.g., vomiting, diarrhoea or constipation), or to the stoma (e.g., peri-stomal infection, buried bumper syndrome). Significant aspiration causing death due to secondary respiratory arrest or peritonitis (after PEG or RIG placement, respectively) has also been reported in the literature (52,53). However, most complications are minor, with reported rates varying between 16% and 70%. Type and frequency of all complications (including mortality) within the first month of insertion are similar, irrespective of the procedure chosen (PEG vs. RIG) (53).
Other symptomatic management
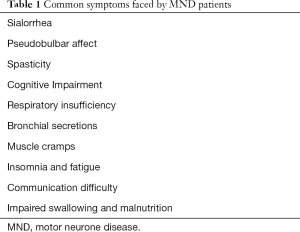
Common symptoms faced by MND patients are summarized in Table 1.
Full table
Sialorrhoea
Excessive salivation is common in MND as bulbar dysfunction worsens and can be embarrassing or result in aspiration. Amitriptyline, atropine, botulin toxin type-b (BTX-B), and external irradiation of the salivary gland have all been tried in the control of sialorrhoea. Costa et al. (54) evaluated the efficacy and safety of BTX-B in the treatment of sialorrhoea in patients with a bulbar onset MND in an open-labelled prospective study that involved the injection of BTX-B into the parotid and submandibular glands. They observed that most patients reported a better QOL while on treatment and a mean reduction of symptom severity of 70%. The most commonly reported side effects include viscous saliva, local pain, chewing weakness, and respiratory infection.
Bronchial secretion
Bulbar impairment results in poor clearance of tenacious sputum. Mucus accumulation is a poor prognostic factor in patients on NIV (55). No RCT exists for any of the treatment approaches in MND. EFNS recommends the use of mucolytic such as N-acetylcysteine when there is sufficient cough flow.
Pseudobulbar affect
This is observed in up to 50% of MND patients (56). Yawning, weeping, and laughing are the characteristic presentation. Pseudobulbar affect has a negative impact on QOL. Small placebo controlled trials and case series have observed the effectiveness of selective serotonin reuptake inhibitors (SSRI) and tricyclic antidepressants in controlling this symptom (57,58). Pioro et al. (59) evaluated the treatment of pseudobulbar affect in patients with multiple sclerosis and MND in a randomized-controlled trial using 30/10 and 20/10 mg dextromethorphan plus ultra-low-dose quinidine. They reported that dextromethorphan plus ultra-low-dose quinidine is effective in reducing the frequency and severity of symptoms and improving patients’ QOL especially at the dose of 30/10 mg combination when compared with placebo. EFNS recommends citalopram (SSRI) and amitriptyline (TCA) for treatment of troublesome cases of pseudobulbar affect (59). A fixed dose combination of dextromethorphan/quinidine (30 mg/30 mg) is the recommended treatment option of AAN (12).
Cramps
Cochrane review found no evidence to recommend any particular treatment in MND-associated cramps, which are a common symptom of the disease (60). Quinine is the most commonly used therapy but its use for cramps is restricted in the USA because of reports of rare but serious haematological and cardiac events (665 serious events and 93 deaths between 1969 and 2006). A further Cochrane review of quinine in patients with various causes of cramp reported some evidence that quinine was safe and effective (60). The adverse effects of quinine are dose-dependent, and the low doses of quinine used in the management of cramps may not necessarily be associated with the serious adverse effects reported in the USA. Alternatively, the studies included in the review could be too small to detect very rare events. In the absence of an effective alternative, the AAN recommends that quinine can be used in MND, but only as a last resort. UK guidelines recommend using quinine as first choice, whereas European guidelines recommend a trial of levetiracetam first, based on the findings of one small study (61). More recently, a small trial, published in 2016, suggested that low-dose mexiletine was well tolerated and reduced the frequency of cramps (61).
It is usually troublesome particularly at night. A RCT failed to support the efficacy of tetracannabinoid in treating moderate to severe cramp (62). A small open-labelled pilot study confirmed that levetiracetam is useful for treatment of cramps in MND patients (62). Modalities such as massage, physical exercise, hydrotherapy, heated pools, and drugs like carbamazepine, diazepam, phenytoin, and verapamil have all been tried without any conclusive evidence. EFNS recommends levetiracetam, quinine sulfate, and physical therapies for management of cramp in MND.
Spasticity
Management of spasticity aims to reduce the impact of increased muscle tone and prevent complications, such as pressure sores or contractures (63-65). Attention should be paid to treating aggravating factors (such as constipation or pain), as well as to posture and seating. Physiotherapy, including passive stretching, can be helpful. One randomized controlled trial of 25 patients indicated that prescribed exercise was associated with small improvements in disability and spasticity (66). Physical therapy is the main treatment modality for spasticity that has its usefulness established from randomized controlled trial in literature (66). Physical therapy methods in use include therapeutic exercise, stretching, positioning, casting, and biofeedback. Other interventions with no controlled trial evidence include heat/cold therapy, hydrotherapy, ultrasound, electrical stimulation, and chemodenervation and rarely surgery can be used (66). Intrathecal Baclofen is the drug of choice in intractable cases (67). Drugs such as Dantrolene, Tetrazepam, and Tizanidine have not been tested in MND in clinical practice but are recommended by EFNS. Nonpharmacological treatment modalities should first be deployed before pharmacological interventions are introduced if symptoms do not improve. These drugs should be used with caution in MND patients as they can cause depression of respiration and worsening of weakness. Physical therapy can be combined with one or more of the antispasticity drugs (68).
Insomnia and fatigue
Insomnia is common in the final stages of MND probably due to cramps, pain, and respiratory impairment (69). Amitriptyline and Zolpidem are some of the medications used in practice without being tested. Fatigue is potentially debilitating and may be of central or peripheral origin. An open-labeled trial confirmed the effectiveness of Modafinil in the treatment of fatigue in MND (70,71).
Cognitive impairment
MND is associated with a frontotemporal type of dementia and it is associated with a negative impact on survival (72). Cognitive impairment has been demonstrated in 20–50% of patients with MND. A number of screening instruments are available for assessing cognitive impairment in MND, but EFNS recommends the use of tools that can assesses verbal fluency as a major component of any test instrument (70).
Communication
It is important for effective social interactions. Subtle changes may be seen as word finding difficulties, spelling difficulty, and decreased verbal output. Language impairment results in difficulties in clinical management and decreases QOL of patients and caregivers (73). EFNS recommends 3–6 monthly assessment; full neuropsychology test; and use of communication aids like computerized speech synthesizer (69).
Supportive and PC
PC and care at the end of life
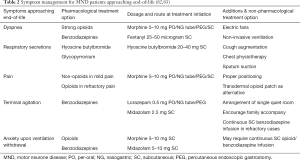
The experience at the end of life can have a significant impact on patients and their families (74-76). Clinical trials in PC have many ethical and practical challenges, and guidance is based on expert consensus (74). Owing to the complexities of the management of advance amyotrophic sclerosis (ALS), guidelines recommend that PC teams should be involved early and throughout the disease (75). In recent literature, role of early PC had shown promising evidence beyond solid malignancies (76). More clinical collaboration and evidence had been established in early PC involvement in advanced organ failure (77), haematological malignancies (78,79) and geriatrics population (62). Patients welcome the ability to participate in advanced care planning, which enables them to discuss and document their attitudes towards future events and medical interventions; however, enactment of these wishes is not always straightforward, and decisions should be reviewed regularly (80,81). Patients’ preferred place of death is usually at home, but distressing symptoms, unanticipated crisis or increasing carer burden can make end-of-life care at home challenging. Advanced planning, good communication and access to expertise in palliative and social care, particularly out of office hours, can avert crises and promote a peaceful death. Anticipatory prescribing of symptomatic therapies in a range of routes (including via gastrostomy, transdermal patches or subcutaneous infusions) is encouraged (82). The Motor Neuron Disease Association (UK) has developed a “Just-in-case” box in which medication can be stored in a patient’s home (83). Carers or clinicians (such as ambulance practitioners or nurses) can then use these medications to treat terminal symptoms. Common symptoms faced by MND patients approaching end-of-life and management options are summarized in Table 2.
Carer support and volunteer service
Informal carers (usually spouses) provide the majority of care for those with MND. This role has a significant negative impact on carers’ physical and emotional wellbeing, burden and QOL (77,84). Carer distress increases with patient distress and with disease progression, and when they are faced with behavioural problems or they lack social and medical support. Carers identify the need for provision of information and training in subjects such as manual handling, as well as the availability of respite care, counselling and access to trained paid-for care (85). PC volunteer roles vary considerably by organization and country as well as over time. It is delivered throughout the world in a wide range of settings, such as purpose-built hospices, day care units, and nursing homes. They assist in psychosocial care, bereavement care, organization of day center activities, hair cutting, musical performance, and engagement in patients’ life review. The roles of volunteers are thought to be complementary to those of professional staff in the care of advanced MND patients. The literature has reported that family caregivers appreciate the emotional support, availability, sustained relationships, and respite that volunteers can provide (86). Role of PC team in the care of advanced MND patients is summarized in Table 3.

Full table
Conclusions
Understanding the specific detail of how best to deliver the interventions described in this review, such as MDC, NIV and nutritional support, is essential to ensure patients derive the benefits (87). Given the clear evidence of benefit of NIV, improving the assessment and optimization of respiratory function should be a priority (88). Another priority should be the effort to evaluate rehabilitative strategies, such as speech therapy programmes and device provision.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rooney J, Byrne S, Heverin M, et al. A multidisciplinary clinic approach improves survival in ALS: a comparative study of ALS in Ireland and Northern Ireland. J Neurol Neurosurg Psychiatry 2015;86:496-501. [Crossref] [PubMed]
- Van den Berg JP, Kalmijn S, Lindeman E, et al. Multidisciplinary ALS care improves quality of life in patients with ALS. Neurology 2005;65:1264-7. [Crossref] [PubMed]
- Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Journal of the Neurological Sciences 1994;124:96-107. [Crossref] [PubMed]
- Khan F, Turner-Stokes L, Ng L, et al. Kilpatrick. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database Syst Rev 2007. [PubMed]
- Greener J, Langhorne P. Systematic reviews in rehabilitation for stroke: issues and approaches to addressing them. Clinical Rehabilitation 2002;16:69-74. [Crossref] [PubMed]
- Turner-Stokes L, Sykes N, Silbert E, et al. From diagnosis to death: exploring the interface between neurology, rehabilitation and palliative care in the management of people with long term neurological conditions. Clin Med (Lond) 2007;7:129-36. [Crossref] [PubMed]
- Hardiman O. Multidisciplinary care in motor neurone disease. In: Kiernan M, editor. Motor Neurone Disease Handbook. Pyrmont: Australasian Medical Publishing Company, 2007.
- Oliver D. Palliative care for motor neurone disease. Pract Neurol 2002;2:68-79. [Crossref]
- Ng L, Khan F, Mathers S. Multidisciplinary care for adults with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev 2009. [PubMed]
- Traynor BJ, Alexander M, Corr B, et al. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996-2000. J Neurol Neurosurg Psychiatry 2003;74:1258-61. [Crossref] [PubMed]
- Cheng HW. Optimizing end-of-life care for patients with hematological malignancy: rethinking the role of palliative care collaboration. J Pain Symptom Manage 2015;49:e5-6. [Crossref] [PubMed]
- Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review). Report of the quality standards subcommittee of the American academy of neurology. Neurology 2009;73:1218-26. [Crossref] [PubMed]
- Andersen PM, Abrahams S, Borasio GD, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)—revised report of an EFNS task force. Eur J Neurol 2012;19:360-75. [Crossref] [PubMed]
- Corcia P, Pradat PF, Salachas F, et al. Causes of death in a postmortem series of ALS patients. Amyotroph Lateral Scler 2008;9:59-62. [Crossref] [PubMed]
- Polkey MI, Green M, Moxham J. Measurement of respiratory muscle strength. Thorax 1995;50:1131-5. [Crossref] [PubMed]
- Morgan RK, McNally S, Alexander M, et al. Use of Sniff nasal-inspiratory force to predict survival in amyotrophic lateral sclerosis. Am J Respir Crit Care Med 2005;171:269-74. [Crossref] [PubMed]
- Hannan LM, Dominelli GS, Chen YW, et al. Systematic review of noninvasive positive pressure ventilation for chronic respiratory failure. Respir Med 2014;108:229-43. [Crossref] [PubMed]
- Kleopa KA, Sherman M, Neal B, et al. Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci 1999;164:82-8. [Crossref] [PubMed]
- Radunovic A, Annane D, Rafiq MK, et al. Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev 2013. [PubMed]
- Pinto A, de Carvalho M, Evangelista T, et al. Nocturnal pulse oximetry: a new approach to establish the appropriate time for noninvasive ventilation in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord 2003;4:31-5. [Crossref] [PubMed]
- Lechtzin N, Scott Y, Busse AM, et al. Early use of noninvasive ventilation prolongs survival in subjects with ALS. Amyotroph Lateral Scler 2007;8:185-8. [Crossref] [PubMed]
- Georges M, Morélot-Panzini C, Similowski T, et al. Noninvasive ventilation reduces energy expenditure in amyotrophic lateral sclerosis. BMC Pulm Med 2014;14:17. [Crossref] [PubMed]
- Gruis KL, Brown DL, Lisabeth LD, et al. Longitudinal assessment of noninvasive positive pressure ventilation adjustments in ALS patients. J Neurol Sci 2006;247:59-63. [Crossref] [PubMed]
- Gonzalez-Bermejo J, Morelot-Panzini C, Arnol N, et al. Prognostic value of efficiently correcting nocturnal desaturations after one month of noninvasive ventilation in amyotrophic lateral sclerosis: a retrospective monocentre observational cohort study. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:373-9. [Crossref] [PubMed]
- Vandenberghe N, Vallet AE, Petitjean T, et al. Absence of airway secretion accumulation predicts tolerance of noninvasive ventilation in subjects with amyotrophic lateral sclerosis. Respir Care 2013;58:1424-32. [Crossref] [PubMed]
- Chiò A, Ilardi A, Cammarosano S, et al. Neurobehavioral dysfunction in ALS has a negative effect on outcome and use of PEG and NIV. Neurology 2012;78:1085-9. [Crossref] [PubMed]
- Gruis KL, Brown DL, Schoennemann A, et al. Predictors of noninvasive ventilation tolerance in patients with amyotrophic lateral sclerosis. Muscle Nerve 2005;32:808-11. [Crossref] [PubMed]
- Bourke SC, Tomlinson M, Williams TL, et al. Effects of noninvasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006;5:140-7. [Crossref] [PubMed]
- Sancho J, Servera E, Díaz JL, et al. Home tracheotomy mechanical ventilation in patients with amyotrophic lateral sclerosis: causes, complications and 1-year survival. Thorax 2011;66:948-52. [Crossref] [PubMed]
- Bach JR. Amyotrophic lateral sclerosis. Communication status and survival with ventilatory support. Am J Phys Med Rehabil 1993;72:343-9. [Crossref] [PubMed]
- Sancho J, Servera E, Bañuls P, et al. Prolonging survival in amyotrophic lateral sclerosis: efficacy of noninvasive ventilation and uncuffed tracheostomy tubes. Am J Phys Med Rehabil 2010;89:407-11. [Crossref] [PubMed]
- Onders RP, Elmo M, Kaplan C, et al. Final analysis of the pilot trial of diaphragm pacing in amyotrophic lateral sclerosis with long-term follow-up: diaphragm pacing positively affects diaphragm respiration. Am J Surg 2014;207:393-7. [Crossref] [PubMed]
- McDermott CJ, Bradburn MJ, Maguire C, et al. Safety and efficacy of diaphragm pacing in patients with respiratory insufficiency due to amyotrophic lateral sclerosis (DiPALS): a multicentre, open-label, randomised controlled trial. Lancet Neurol 2015;14:883-92. [Crossref] [PubMed]
- Tulsky DS, Rosenthal M. Measurement of quality of life in rehabilitation medicine: emerging issues. Archives of Physical Medicine and Rehabilitation 2003;84:S1-2. [Crossref] [PubMed]
- Hadjikoutis S, Wiles CM, Eccles R. Cough in motor neuron disease: a review of mechanisms. QJM 1999;92:487-94. [Crossref] [PubMed]
- Bach JR. Amyotrophic lateral sclerosis: prolongation of life by noninvasive respiratory aids. Chest 2002;122:92-8. [Crossref] [PubMed]
- Bach JR, Ishikawa Y, Kim H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest 1997;112:1024-8. [Crossref] [PubMed]
- Brito MF, Moreira GA, Pradella-Hallinan M, et al. Air stacking and chest compression increase peak cough flow in patients with Duchenne muscular dystrophy. J Bras Pneumol 2009;35:973-9. [Crossref] [PubMed]
- Toussaint M, Boitano LJ, Gathot V, et al. Limits of effective cough-augmentation techniques in patients with neuromuscular disease. Respiratory Care 2009;54:359-66. [PubMed]
- Ahmed RM, Irish M, Piguet O, et al. Amyotrophic lateral sclerosis and frontotemporal dementia: distinct and overlapping changes in eating behaviour and metabolism. Lancet Neurol 2016;15:332-42. [Crossref] [PubMed]
- Jesse S, Thal DR, Ludolph AC. Thiamine deficiency in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2015;86:1166-8. [Crossref] [PubMed]
- Dupuis L, Corcia P, Fergani A, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 2008;70:1004-9. [Crossref] [PubMed]
- Kasarskis EJ, Berryman S, Vanderleest JG, et al. Nutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of death. Am J Clin Nutr 1996;63:130-7. [Crossref] [PubMed]
- Desport JC, Preux PM, Truong TC, et al. Nutritional status is a prognostic factor for survival in ALS patients. Neurology 1999;53:1059-63. [Crossref] [PubMed]
- Desport JC, Preux PM, Truong CT, et al. Nutritional assessment and survival in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:91-6. [Crossref] [PubMed]
- Desport JC, Torny F, Lacoste M, et al. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neurodegener Dis 2005;2:202-7. [Crossref] [PubMed]
- NCGC. Motor neurone disease: assessment and management. In: National Institute for Health and Care Excellence: Clinical Guidelines. London: National Institute for Health and Care Excellence (UK), 2016.
- Chiò A, Galletti R, Finocchiaro C, et al. Percutaneous radiological gastrostomy:a safe and effective method of nutritional tube placement in advanced ALS. J Neurol Neurosurg Psychiatry 2004;75:645-7. [Crossref] [PubMed]
- Chavada G, El-Nayal A, Lee F, et al. Evaluation of two different methods for per-oral gastrostomy tube placement in patients with motor neuron disease. (MND): PIG versus PEG procedures. Amyotroph Lateral Scler 2010;11:531-6. [Crossref] [PubMed]
- Laasch HU, Wilbraham L, Bullen K, et al. Gastrostomy insertion: comparing the options - PEG, RIG or PIG? Clin Radiol 2003;58:398-405. [Crossref] [PubMed]
- Verschueren A, Monnier A, Attarian S, et al. Enteral and parenteral nutrition in the later stages of ALS: an observational study. Amyotroph Lateral Scler 2009;10:42-6. [Crossref] [PubMed]
- Thornton FJ, Fotheringham T, Alexander M, et al. Amyotrophic lateral sclerosis: enteral nutrition provision - endoscopic or radiologic gastrostomy? Radiology 2002;224:713-7. [Crossref] [PubMed]
- Desport J-C, Mabrouk T, Bouillet P, et al. Complications and survival following radiologically and endoscopically-guided gastrostomy in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2005;6:88-93. [Crossref] [PubMed]
- Costa J, Rocha ML, Ferreira J, et al. Botulinum toxin type-B improves sialorrhea and quality of life in bulbaronset amyotrophic lateral sclerosis. J Neurol 2008;255:545-50. [Crossref] [PubMed]
- Peysson S, Vandenberghe N, Philit F, et al. Factors predicting survival following noninvasive ventilation in amyotrophic lateral sclerosis. Eur Neurol 2008;59:164-71. [Crossref] [PubMed]
- Gallagher JP. Pathologic laughter and crying in ALS: a search for their origin. Acta Neurologica Scandinavica 1989;80:114-7. [Crossref] [PubMed]
- Szczudlik A, Słowik A, Tomik B. The effect of amitriptyline on the pathological crying and other pseudobulbar signs. Neurologia i Neurochirurgia Polska 1995;29:663-74. [PubMed]
- Iannaccone S, Ferini-Strambi L. Pharmacologic treatment of emotional lability. Clinical Neuropharmacology 1996;19:532-5. [Crossref] [PubMed]
- Pioro EP, Brooks BR, Cummings J, et al. Dextromethorphan plus ultra-low-dose quinidine reduces pseudobulbar affect. Annals of Neurology 2010;68:693-702. [Crossref] [PubMed]
- El-Tawil S, et al. Quinine Muscle Cramps. Cochrane Database Syst Rev 2015. [PubMed]
- Bedlack RS, Pastula DM, Hawes J, et al. Open-label pilot trial of levetiracetam for cramps and spasticity in patients with motor neuron disease. Amyotroph. Lateral Scler 2009;10:210-5. [Crossref] [PubMed]
- Cheng HW, Li CW, Chan KY, et al. Bringing palliative care into geriatrics in a Chinese culture society--results of a collaborative model between palliative medicine and geriatrics unit in Hong Kong. J Am Geriatr Soc 2014;62:779-81. [Crossref] [PubMed]
- Katzberg HD, Khan AH, So YT. Assessment: symptomatic treatment for muscle cramps (an evidence-based review): report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2010;74:691-6. [Crossref] [PubMed]
- Ashworth NL, Satkunam LE, Deforge D. Treatment for spasticity in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev 2012. [PubMed]
- Kheder A, Nair KP. Spasticity: pathophysiology, evaluation and management. Pract Neurol 2012;12:289-98. [Crossref] [PubMed]
- Drory VE, Goltsman E, Reznik JG, et al. The value of muscle exercise in patients with amyotrophic lateral sclerosis. J Neurol Sci 2001;191:133-7. [Crossref] [PubMed]
- McClelland S III, Bethoux FA, Boulis NM, et al. Intrathecal baclofen for spasticity-related pain in amyotrophic lateral sclerosis: efficacy and factors associated with pain relief Muscle & Nerve 2008;37:396-8. [Crossref] [PubMed]
- Carter GT, Miller RG. Comprehensive management of amyotrophic lateral sclerosis. Phys Med Rehabil Clin N Am 1998;9:271-84. [Crossref] [PubMed]
- Lou JS. Fatigue in amyotrophic lateral sclerosis. Phys Med Rehabil Clin N Am 2008;19:533-43. [Crossref] [PubMed]
- Carter GT, Weiss MD, Lou JS, et al. Modafinil to treat fatigue in amyotrophic lateral sclerosis: an open label pilot study. Am J Hosp Palliat Care 2005;22:55-9. [Crossref] [PubMed]
- Rabkin JG, Gordon PH, Mcelhiney M, et al. Modafinil treatment of fatigue in patients with ALS: a placebo-controlled study. Muscle and Nerve 2009;39:297-303. [Crossref] [PubMed]
- Gordon PH, Cheng B, Katz IB, et al. The natural history of primary lateral sclerosis. Neurology 2006;66:647-53. [Crossref] [PubMed]
- Cobble M. Language impairment in motor neurone disease. J Neurol Sci 1998;160:S47-S52. [Crossref] [PubMed]
- Whitehead B, O’Brien MR, Jack BA, et al. Experiences of dying, death and bereavement in motor neurone disease: A qualitative study. Palliat. Med 2012;26:368-78. [Crossref] [PubMed]
- Aoun SM, Connors SL, Priddis L, et al. Motor Neurone Disease family carers’ experiences of caring, palliative care and bereavement: an exploratory qualitative study. Palliat Med 2012;26:842-50. [Crossref] [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Chan KY, Yip T, Yap DYH, et al. Enhanced psychosocial support for caregiver burden for patients with chronic kidney failure choosing not to be treated by dialysis or transplantation: a pilot randomized controlled trial. Am J Kidney Dis 2016;67:585-92. [Crossref] [PubMed]
- Cheng HW, Li CW, Chan KY, et al. End-of-life characteristics and palliative care provision for elderly patients suffering from acute myeloid leukemia. Support Care Cancer 2015;23:111-6. [Crossref] [PubMed]
- Cheng BHW, Sham MM, Chan KY, et al. Intensive palliative care for patients with hematological cancer dying in hospice: analysis of the level of medical care in the final week of life. Am J Hosp Palliat Care 2015;32:221-5. [Crossref] [PubMed]
- Baxter SK, Baird WO, Thompson S, et al. The use of non-invasive ventilation at end of life in patients with motor neurone disease: A qualitative exploration of family carer and health professional experiences. Palliat Med 2013;27:516-23. [Crossref] [PubMed]
- Phelps K, Regen E, Oliver D, et al. Withdrawal of ventilation at the patient’s request in MND: a retrospective exploration of the ethical and legal issues that have arisen for doctors in the UK. BMJ Support Palliat Care 2017;7:189-96. [Crossref] [PubMed]
- National Clinical Guideline Centre. Care of Dying Adults in the Last Days of Life. 2015. Available online: www.nice.org.uk/guidance/ng31?unlid=8981581202016219221856
- Motor Neurone Disease Association. MND Just in Case kit - information for professionals. Available online: www.mndassociation.org/forprofessionals/mndmanagement/mnd-just-in-case-kit/
- Peters M, Jenkinson C, Doll H, et al. Carer quality of life and experiences of health services: a cross-sectional survey across three neurological conditions. Health Qual Life Outcomes 2013;11:103. [Crossref] [PubMed]
- O’Brien MR, Whitehead B, Jack BA., et al. The need for support services for family carers of people with motor neurone disease (MND): views of current and former family caregivers a qualitative study. Disabil Rehabil 2012;34:247-56. [Crossref] [PubMed]
- Cheng HW, Li CW, Chan KY, et al. The first confirmed case of human avian influenza A(H7N9) in Hong Kong and the suspension of volunteer services: impact on palliative care. J Pain Symptom Manage 2014;47:e5-7. [Crossref] [PubMed]
- Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [Crossref] [PubMed]
- Cheng HW, Chen WT, Chu CK, et al. The development of neurology palliative care service for motor neuron disease (MND) patients: Hong Kong experience. Ann Palliat Med 2017. [Epub ahead of print]. [Crossref] [PubMed]