Longevity after radiotherapy of stage III lung cancer: superior vena cava obstruction is associated with early mortality
Introduction
The incidence of stage III lung cancer is approximately 30% in a population-based cancer registry (1). The estimated overall 5-year survival rates are 14% to 25% for stage IIIA and 5% to 9% for stage IIIB non-small cell lung cancers (2). Although the prognosis for patients with locally advanced lung cancer (LALC) is not often in doubt, life expectancies (LE) can vary from days to years in this population. Available information is limited with regard to descriptions of life spans following radiation treatment of LALC. Moreover, there have been few published studies exploring influential factors associated with different life spans. It is in this context that we defined several periods of survival and attempted to characterize each category of longevity.
Methods
After obtaining approval from the institutional review board, an audit of the radiation oncology records was undertaken, and the cancer center database was queried for histologically-proven cases of lung cancer. Our retrospective review yielded a study population of 133 consecutive individuals treated between September 1981 and August 2010 for stage IIIA or IIIB lung cancer as defined in the revised American Joint Committee on Cancer (AJCC) staging system (3). All patients underwent pre- and post-treatment imaging studies (chest radiograph, computed tomography or positron emission tomography). Survival analysis showed that 25 people (19%) survived for 3 months or less; they comprised the end-of-life (EOL) group. Survival ranged from 4 to 36 months in 94 patients (71%), and they represented the short-term life span (STLS) cohort. Fourteen individuals (10%) had life spans between 44 months and 163 months; these individuals composed the long-term survival (LTS) category.
At our cancer center, concurrent chemoradiation for stage III lung cancer is usually practiced, but in five patients chemotherapy was sequentially administered because of poor performance status, superior vena cava obstruction (SVCO), small cell lung cancer histology or frail habitus. The chemotherapy treatment scheme involved the intravenous administration of cisplatin (50 mg/m2 given on days 1, 8, 29 and 36) and etoposide (50 mg/m2 given on days 1 to 5 and 29 to 33) for 4 cycles. The thoracic radiotherapy was megavoltage external beam fractionated irradiation. When the treatment intent was definitive, the total prescription dose was ≥60 Gy, given in 30 to 33 fractions; after 40 Gy, the spinal cord was excluded from irradiation. When the intrathoracic disease was considered too extensive for curative chemoradiotherapy, the treatment aim was for disease palliation, and the dose did not exceed 50 Gy; 30 Gy given in 10 fractions was usually applied. The supraclavicular area was included in the irradiated field when the primary lesion was located in the upper lobe or when suspected mediastinal or supraclavicular metastatic disease was demonstrated.
Follow-up data were obtained from a prospectively maintained, computerized database of the cancer center and from radiation oncology records. Clinical and radiological evaluations after treatment were performed by one or several staff physicians. The average interval from completion of radiotherapy to the first surveillance imaging was 3.6 months. Determinations of tumor status (regression, stasis, or growth) were made through side-by-side comparison of similar pre- and post-treatment imaging; the reviews were performed by the radiation oncologists in older cases and by the medical oncologists or pulmonary physicians during the later years of the study. Generally, after-treatment clinical examinations were requested at 3-month intervals during the first year, at 6-month intervals for the next 4 years, and annually thereafter. Tumor responses were evaluated and were scored according to the Response Evaluation Criteria in Solid Tumors (RECIST) method (4). A complete response was defined as the complete disappearance of the neoplastic lesion; a reduction in tumor size of at least 30% was considered a partial response. Progressive disease was indicated by an increase of at least 20% in the size of the lesion.
Survival was measured from the time of diagnosis of LALC until death or last contact. The Kaplan-Meier method and log-rank test were utilized to calculate and compare the survival rates associated with the three classes of longevity. Possible prognostic factors such as age, Eastern Cooperative Oncology Group performance status score, AJCC stage III subgroup classifications, tumor histologic subtypes, the presence or absence of comorbid illness or clinically manifested SVCO, the administration or omission of chemotherapy, the intent of radiotherapy (definitive or palliative), and the possession of adequate, inadequate or no health insurance were evaluated by comparative univariate analysis (chi-square or Fisher’s test). The resulting significant factors were then tested, employing the multiple regression model, for their potential as independent predictors of short (≤3 months) survival. SAS version 9.4 (Cary, NC) was used for statistical computing, and P<0.05 was considered statistically significant.
Results
Patient and treatment characteristics
Out of 133 people in our study, 81 (61%) were men, and 52 (39%) were women. Individuals were classified according to specific age groups. There were two patients in the <20–30 years category, three patients in the 31–40 years category, 18 patients in the 41–50 years category, 56 patients in the 51–64 years category, and 54 patients classified as elderly (older than 64 years). Table 1 shows that many individuals presented with an acceptable Eastern Cooperative Oncology Group performance status score of 0–1, (71%; 94 patients), stage IIIB disease (66%; 88 patients) and comorbid illnesses (70%; 93 patients). The majority of lung cancer cases were not associated with clinically manifested SVCO (68%; 90 patients) and were of the non-small cell tumor subtype (86%; 115 patients). Of the 43 individuals with SVCO, 38 patients received palliative radiotherapy (PRT), two patients were treated with radical radiotherapy and three patients underwent radical chemoradiotherapy. Most of the studied subjects were uninsured or underinsured (79%; 105 patients). Furthermore, close to half of the individuals were non-elderly (59%; 79 patients) and received chemotherapy (52%; 69 patients) and definitive radiotherapy (51%; 68 patients). Among the 52 evaluable of the 133 individuals, the tumor response rate was complete, partial or absent in 17%, 77% and 6% of the cases, respectively. Subsequent disease progression occurred in 69% of the assessed patients. At the time of analysis, 12 patients were alive during a median follow-up of 55.5 (range, 25–163) months. There were 121 deceased individuals, and the median survival in this group was 10 months. The overall crude survival rates at 1, 2, 3 and 5 years were 36%, 14%, 7% and 2%, respectively.
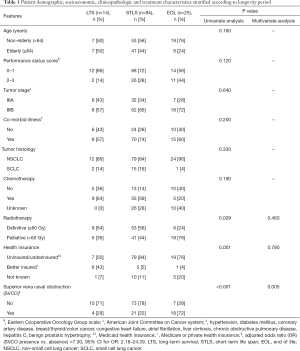
Full table
Longevity periods and characterization analysis
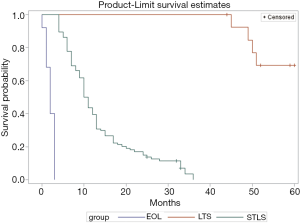
The three longevity categories with corresponding survival rates are shown in Figure 1 (P<0.001). As disclosed in Table 1, the non-small cell tumor histologic type and the presence of comorbid illness were found among most patients in the three longevity classes. Possession of better health insurance was more common among long-term survivors than EOL or STLS patients (P=0.001). Overall, there were only 12 patients with better insurance; none of these people received earlier care. Among the 14 long-term survivors, only six patients possessed better health insurance; among these, five individuals were treated with higher doses of radiation, and one subject received a lower dose. Also, two patients were diagnosed with stage IIIA disease, while four patients were diagnosed with stage IIIB lung cancer. Despite the fact that more of the younger patients were present in the EOL longevity category than in the other two classified longer life groups, those of the EOL cohort presented with a poorer performance status and more ominous stage IIIB disease. Moreover, fewer EOL subjects received chemotherapy and definitive radiotherapy. None of these observations with a negative implication potential reached statistical significance. On univariate analysis (Table 1), factors significantly predictive of poor prognosis were the presence of SVCO (P<0.001), receipt of PRT (P=0.009) and the possession of inadequate or no health insurance (P=0.001). However, on multivariate analysis, the only independent clinical characteristic indicating shortened survival/EOL status was the occurrence of SVCO (P=0.005).
Discussion
An accurate estimate of prognosis in individuals with advanced cancer is important to avoid futile therapy and unnecessary toxicity. Effective treatment applied to an a priori unfavorable cohort of patients may be erroneously interpreted as lacking benefit. We undertook this investigation because there are people who may survive longer than others, people whose LE is extremely short despite the application of standard of care therapeutic intervention, and people who are not suitable for state-of-the-art radical treatment but who are also judged not to have a very short life. Some physicians manage the disease in this last patient group with only a palliative intent, whereas others advocate a more intensive treatment regimen.
In this study, STLS represented the predominant longevity pattern after radiotherapy of LALC compared to the LTS or EOL situation, and this particular cohort proved difficult to characterize. From the literature, there is little information in this regard. We found that only about 10% of the studied subjects were long-term survivors [an observation similar to the findings in other reports about people with treated lung cancer (1,5,6)]. Possession of better health insurance might have contributed to the improved prognosis, considering that most of these people received chemotherapy and definitive radiotherapy. In an investigation (7) to determine the influence of health insurance status on the outcome of 299,914 patients with non-small cell lung cancer registered in the National Cancer Database, payer status was found to be a significant predictor of overall survival. The uninsured and Medicaid patients had an increased risk of dying compared to those individuals with Medicare; the observed mortality rates were 36%, 21% and 17%, respectively. Forrest and colleagues (8) conducted a systematic review of the literature to determine whether socioeconomic inequalities in treatment occur and whether such circumstances affect mortality in people with lung cancer. The conclusion derived from the meta-analysis was that patients living in more deprived conditions were less likely to receive any type of therapy. In other studies of lung cancer patients treated with curative or palliative intent, a common clinical characteristic indicative of 5-year survival was the presentation of better performance status at the time of diagnosis and treatment (5,9,10).
Early mortality after radiotherapy for LALC is not uncommon. In the present experience, the 19% incidence of individuals in their last 3 months of life post-irradiation is higher than the 5% to 15% rate of occurrence noted by other investigators (11-14). Recent reports showed that early mortality was associated with poor performance status, increasing age, the presence of dyspnea, lower albumin and higher lactic acid dehydrogenase levels (11-17). In our view, EOL, as defined here and in several studies (11-19), indicates a phase in which directed therapy is no longer possible and the patient’s condition declines. A determination of the reasons for early death is warranted so that ways to decrease such occurrences may be developed. During this period, symptom burden will increase and in the end, will become high. The quality of EOL care delivered to patients who die as a result of cancer is a major public health concern. It is not clear whether any treatment should be considered as a marker of poor quality EOL care. The literature (12,15,17,18) has shown that a significant proportion of patients died within a period of time too short to experience the additional benefit of PRT. Hence, it is believed that therapy applied in the last days or month of life is likely to provide minimal palliation or survival benefit. As a result, the concept of percentage of remaining life spent while receiving PRT has been proposed as a possible quality indicator of EOL care. Moreover, understanding what is important in the patient’s terminal period is integral to the success of improving the care of the dying. In a study to determine factors considered important at the EOL, Steinhauser et al. (20) found that the participants ranked freedom from pain and dying at home as the most and least important, respectively. In the EOL situation, hospice has become the paradigm for high quality care because system-enrolled patients apparently consume less health care resources and generate less health care expenditures.
Our study demonstrated that the majority of patients experienced STLS following conventional treatment of LALC and also determined that SVCO is an independent predictor of shortened survival. Martins and Pereira (21) evaluated the correlation of clinical characteristics to survival in 1,635 patients with non-small cell lung cancer treated in Brazil. In their experience, the presence of superior vena cava syndrome (SVCS) in individuals with stage III disease was associated with a poor prognosis. Similarly, Gauden (22) retrospectively reviewed 249 patients treated by radiation for lung cancer-associated SVCS at the Queensland Radium Institute and found that the overall 2-year survival rate for the group was 5%. Our study has acknowledged limitations. Major drawbacks include the lack of documentation of clinical responses of SVCO to treatment and an inherent selection bias because this investigation was conducted in a retrospective fashion and all treatments were given at a single institution. We recognize that the approach to management of LALC evolved during the long period of our described experience. Currently, it is widely accepted that chemoradiotherapy has better value over radiotherapy alone, and that chemotherapy should be administered concurrently instead of sequential administration. Technological advances in radiotherapy have included intensity modulated radiation therapy; this improved technique of photon irradiation has enabled treatments with higher conformity and has allowed dose escalation to target areas with gross disease. Another limitation of our study is that these two contemporary management innovations were not used in most of our study participants. In our view, more evidence is needed to show the usefulness of molecular therapy, especially the prognostic implication of molecular features such as EGFR, ALK, PDL1. Also, how to select lung cancer patients with metastases who might derive benefit from the early administration of therapy remains to be clarified.
Conclusions
LALC is generally an incurable disease condition. LE is a key to determine whether a patient has sufficient longevity to benefit from treatment. Because of consistent physician inaccuracy of LE estimates, several prognostic models have been developed to provide a more accurate prediction of life span of patients with advanced stage cancer. These prognostic indices, however, have been restricted to characterization and correlated with median survival. Reviewing long-standing practice, especially about different periods of survival after treatment, may constitute an important step towards measuring and improving outcomes of lung cancer care, especially to those who are near the EOL. Individual outcome is variable, and even the most favorable patient group contains people with very short, intermediate or long LE. Therefore, identification of longevity predictive features in LALC patients deserves further scrutiny.
Acknowledgements
None.
Footnote
Conflicts of Interest: This study was presented in part at the American Thoracic Society International Conference. May 19–24, 2017; Washington, D.C.
Ethical Statement: The study was approved by LSU Health Sciences Center-Shreveport Institutional Review Board (NO. 0347).
References
- Wang BY, Huang JY, Cheng CY, et al. Lung cancer and prognosis in Taiwan: A population-based cancer registry. J Thorac Oncol 2013;8:1128-35. [Crossref] [PubMed]
- Brower JV, Amini A, Chen S, et al. Improved survival with dose-escalated radiotherapy in stage III non-small-cell lung cancer: analysis of the National Cancer Database. Ann Oncol 2016;27:1887-94. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th edition. Springer, 2010.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. JNCI 2000;92:205-16. [Crossref] [PubMed]
- Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. JNCI 1996;88:1210-5. [Crossref] [PubMed]
- Ohe Y, Ishizuka N, Tamura T, et al. Long-term follow-up of patients with unresectable locally advanced non-small cell lung cancer treated with chemoradiotherapy: A retrospective analysis of the data from the Japan Clinical Oncology Group trials (JCOG0003A). Cancer Sci 2003;94:729-34. [Crossref] [PubMed]
- Shi R, Diaz R, Shi Z, et al. The effect of payer status on survival of patients with stage I/II non-small cell lung cancer: NCDB 1998-2011. AntiCancer Res 2016;36:319-26. [PubMed]
- Forrest LF, Adams J, Wareham H, et al. Socioecnomic inequalities in lung cancer treatment: Systematic review and meta-analysis. PLOS Med 2013;10:e1001376. [Crossref] [PubMed]
- Komaki R, Cox JD, Hartz J, et al. Characteristics of long-term survivors after treatment for inoperable carcinoma of the lung. Am J Clin Oncol 1985;8:362-70. [Crossref] [PubMed]
- MacManus MP, Matthews JP, Wada M, et al. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer 2006;106:1110-6. [Crossref] [PubMed]
- Arkenau HT, Olmos D, Ang JE, et al. 90-days mortality rate in patients treated within the context of a phase-I trial: How should we identify patients who should not go on trial? Eur J Cancer 2008;44:1536-40. [Crossref] [PubMed]
- Gripp S, Mjartan S, Boelke E, et al. Palliative radiotherapy tailored to life expectancy in end-stage cancer patients: Reality or myth? Cancer 2010;116:3251-6. [Crossref] [PubMed]
- Groome PA, Schulze KM, Keller S, et al. Demographic differences between cancer survivors and those who die quickly of their disease. Clin Oncol (R Coll Radiol) 2008;20:647-56. [Crossref] [PubMed]
- van Oorschot B, Assenbrunner B, Schuler M, et al. Survival and prognostic factors after moderately hypofractionated palliative thoracic radiotherapy for non-small cell lung cancer. Strahlenther Onkol 2014;190:270-5. [Crossref] [PubMed]
- Berger B, Ankele H, Bamberg M, et al. Patients who die during palliative radiotherapy. Strahlenther Onkol 2014;190:217-20. [Crossref] [PubMed]
- Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: The TEACHH Model. Cancer 2014;120:134-41. [Crossref] [PubMed]
- Toole M, Lutz S, Johnstone PA. Radiation oncology quality: Aggressiveness of cancer care near the end of life. J Am Coll Radiol 2012;9:199-202. [Crossref] [PubMed]
- Kapadia NS, Mamet R, Zornosa C, et al. Radiation therapy at the end of life in patients with incurable nonsmall cell lung cancer. Cancer 2012;118:4339-45. [Crossref] [PubMed]
- Li D, Prigerson HG, Kang J, et al. Impact of radiation therapy on aggressive care and quality of life near death. J Pain Symptom Manage 2017;53:25-32. [Crossref] [PubMed]
- Steinhauser KE, Christakis NA, Clipp EC, et al. Factors considered important at the end of life by patients, family physicians, and other care providers. JAMA 2000;284:2476-82. [Crossref] [PubMed]
- Martins SJ, Pereira JR. Clinical factors and survival in non-small cell lung cancer. Am J Clin Oncol 1999;22:453-7. [Crossref] [PubMed]
- Gauden SJ. Superior vena cava syndrome induced by bronchogenic carcinoma: Is this an oncological emergency? Australas Radiol 1993;37:363-6. [Crossref] [PubMed]


