Symptom clusters using the EORTC QLQ-C15-PAL in palliative radiotherapy
Introduction
It has been long acknowledged that patients with advanced cancer experience a multitude of symptoms, either due to their disease or treatment (1). Due to the potential interrelation of symptoms, analyses have revealed symptom clusters consisting of 2 or more concurrent symptoms in certain patient populations. Dodd et al. were among the first to demonstrate the clinical significance of this phenomenon, as they provided early insights into the effects of symptom clusters on individual functional status (2). Further research, as well as knowledge dissemination and application of symptom clusters has been greatly advocated for in the palliative care (3).
In oncological research, the Edmonton Symptom Assessment System (ESAS), M.D. Anderson Symptom Inventory and Symptom Distress Scale are commonly used (4). Although identification and validation of symptom clusters in palliation can have profound implications on care in this setting, there have been few studies conducted reporting on clusters using tools specific to palliative patient populations. The present study investigated symptom clusters in patients undergoing radiotherapy for advanced cancer using a palliative-specific quality of life (QoL) assessment tool.
Methods
Patients undergoing palliative radiotherapy at the Rapid Response Radiotherapy Program (RRRP) in Sunnybrook Health Sciences Centre were enrolled in two clinical trials testing the safety and efficacy of anti-emetics ondansetron (5) and palonosetron in the prophylaxis of radiation-induced nausea and vomiting. Ethics approval was obtained, and all patients provided informed consent. Patients received radiotherapy to bone metastases or soft tissue masses in the lower abdomen and pelvic regions. QoL was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life-C15-Palliative (EORTC QLQ-C15-PAL) at baseline and 2 follow-up intervals (days 5 and 10 post-radiation). Validated for use in patients with advanced cancer, the C15-PAL assesses various aspects of QoL with 14 items (Q1–14) on a 4 point Likert scale (1—not at all, to 4—very much), and overall QoL (Q15) (6). In the present study, analyses were conducted using responses for Q1–14.
Patient demographics (e.g., age, gender), disease-related characteristics (e.g., primary cancer, site of metastases) and treatment-related characteristics (e.g., dose/fractions) were included. To compare baseline characteristics between the two studies, a Wilcoxon rank-sum nonparametric test or Fisher exact test was applied for continuous and categorical variables. Two-sided P values of less than 0.05 were considered statistically significant.
Symptom clusters were identified using three different statistical procedures: the principal component analysis (PCA), exploratory factor analysis (EFA), and hierarchal cluster analysis (HCA).
PCA
To examine whether any interrelationships existed between the items, PCA for qualitative data was performed for Q1–14 at baseline using the PRINQUAL procedure in statistical analysis software (SAS version 9.4 for Windows). The PCA transforms ordinal variables monotonically by scoring the ordered categories, so that the covariance matrix is optimized (7). The PRINQUAL procedure iterations produce a set of transformed variables. Each symptom’s new scoring satisfies a set of constraints based on the original scoring of the symptom and the specified transformation type. The new set of scores is selected from the sets of possible scorings that do not violate the constraints so that the method criterion is locally optimized, and the varimax rotation was also applied.
The first principal component accounts for as much of the variability in the data as possible. The number of significant principal components was selected with an eigenvalue higher than 1.0; wherein, each component explained at least 5% of the variance, and contained at least two symptoms. The highest factor loading score predicted the assignment of individual symptoms to an independent factor. The internal consistency and reliability of the derived clusters was assessed with Cronbach’s alpha (<0.5 as unacceptable, ≥0.5 as poor, ≥0.6 as questionable, ≥0.7 as acceptable, ≥0.8 as good, and ≥0.9 as excellent internal consistency) (8,9)
EFA
The EFA is the most commonly applied method of cancer symptom cluster identification (10,11). Applied to this area of symptom cluster research, factor analysis is a statistical approach used for finding the common factors that explain the correlation between symptoms and, extending this conceptually, finding the commonality that ‘‘binds’’ 2 or more symptoms together into a common concept (12). Factor analysis is used to predict a set of latent factors that are responsible for covariance among a group of symptoms. Symptoms due to this latent factor would covary more strongly with each other than they would with symptoms that are affected by a different latent factor (13).
With the EFA, the maximum likelihood method was applied for approximately multivariate normal data. The varimax orthogonal rotation method was also used. The numbers of factors were also selected by the eigenvalue greater than 1.0, indicating that almost 10% of variance in the symptom is shared with the latent factor after controlling for the correlation between factors. PROC FACTOR procedure in SAS was conducted for this analysis.
HCA
The HCA is another procedure that can be used to define a symptom cluster. Cluster analysis is an exploratory technique that it is used to discover underlying groups of individuals who are similar in their symptom experience or symptom profile (10). Therefore, this method is focused on classification and trying to put similar entities together into a cluster and separate this cluster from other clusters. The PROC VARCLUS run clusters on the basis of centroid components.
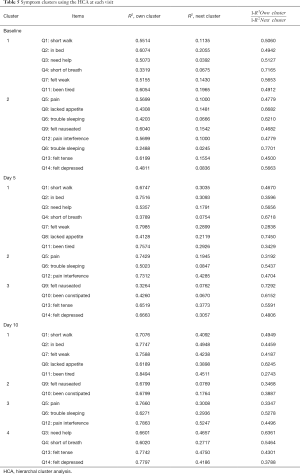
The centroid cluster algorithm was first used to split the variables into the two clusters. 1−R2 ratio was the ratio of one minus the value in the Own Cluster column to one minus the value in the Next Closest column. Cluster analyses were conducted additionally until low ratios indicated well-separated clusters.
Results
There were 109 patients with complete C15-PAL data at baseline who were included in the present analysis. The average age was 72 years, and the majority of patients were male (67.9%) (Table 1). The most common primary was prostate (36.7%), and almost all included patients presented with bone metastases (95.4%).
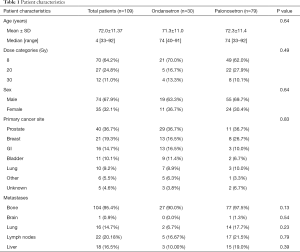
Full table
Among the 109 total patients who had baseline C15-PAL scores, 90 and 87 patients had complete C15-PAL data at days 5 and 10 respectively. C15-PAL scores for Q1–14 at each visit are summarized in Table 2. Different symptom clusters were identified at the three time points. Factor loadings and final communalities using the PCA and EFA are provided in Tables 3,4, respectively. R2 figures for own and next clusters, as well as 1−R2 ratios used in the HCA are provided in Table 5.
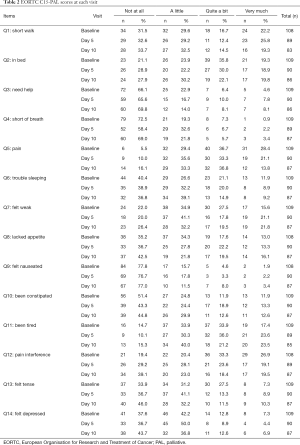
Full table
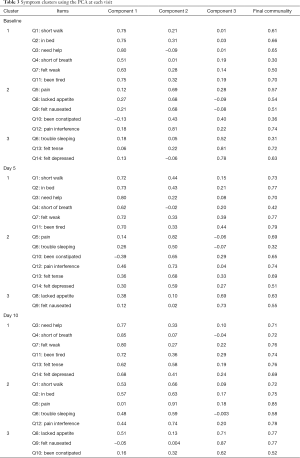
Full table
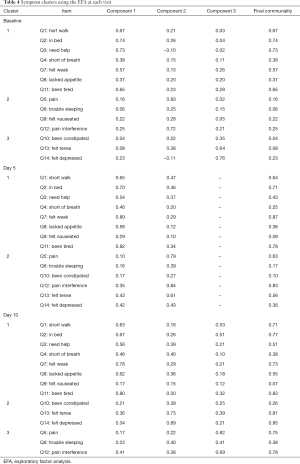
Full table
Full table
Baseline
Using the PCA, 3 clusters were observed (component 1: Q1–4, Q7, Q11; component 2: Q5, Q8–10, Q12; component 3: Q6, Q13–14). The clusters accounted for 32.3%, 12.8% and 10.4% of the total variance; and had Cronbach’s alpha values of 0.81, 0.65 and 0.53, respectively. The EFA method identified 3 clusters (component 1: Q1–4, Q7–8, Q11; component 2: Q5–6, Q9, Q12; component 3: Q10, Q13–14). The clusters explained 61.4%, 21.6% and 17.0% of the total variance; and had Cronbach’s alpha values of 0.81, 0.61 and 0.60, respectively. The HCA derived 3 clusters (cluster 1: Q1–4, Q7, Q11; cluster 2: Q5, Q8–9, Q12; cluster 3: Q6, Q10, Q13–14). The clusters accounted for 52%, 50% and 42% of the total variance, respectively.
Day 5 follow-up
The PCA produced 3 clusters (component 1: Q1–4, Q7, Q11; component 2: Q5–6, Q10, 12–14; component 3: Q8–9). The clusters accounted for 45.5%, 11.4%, and 7.2% of the total variance; and had Cronbach’s alpha values of 0.90, 0.80 and 0.55, respectively. The EFA method identified 2 clusters (component 1: Q1–4, Q7–9, Q11; component 2: Q5–6, Q10, Q12–14). The two clusters explained 86.4% and 13.6% of the total variance; and had Cronbach’s alpha values of 0.87 and 0.80, respectively. HCA derived 3 clusters (cluster 1: Q1–4, Q7–8, Q11; cluster 2: Q5–6, Q12; cluster 3: Q9–10, Q13–14). The clusters explained 60%, 65% and 51% of the total variance, respectively.
Day 10 follow-up
The PCA identified 3 clusters (component 1: Q3–4, Q7, Q11, Q13–14; component 2: Q1–2, Q5–6, Q12; component 3: Q8–10). The clusters accounted for 54.0% 10.2% and 8.0% of the total variance; and had Cronbach’s alpha values of 0.90, 0.88 and 0.65, respectively. The EFA method derived 3 clusters (component 1: Q1–4, Q7–9, Q11; component 2: Q10, Q13–14; component 3: Q5–6, Q12). These clusters explained 81.7%, 12.6% and 5.7% of the total variance; and had Cronbach’s alpha values of 0.87, 0.72 and 0.73, respectively. The HCA derived 4 clusters (cluster 1: Q1–2, Q7–8, Q11; cluster 2: Q9–10; cluster 3: Q5–6, Q12; cluster 4: Q3–4, Q13–14). The 4 clusters accounted for 74.0%, 68.0%, 72.5% and 70.2% of the total variance.
Discussion
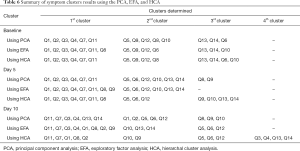
A summary of the symptom clusters identified using the three statistical procedures at all time points are displayed in Table 6. At baseline, items “short walk” (Q1), “in bed” (Q2), “need help” (Q3), “short of breath” (Q4), “felt weak” (Q7) and “been tired” (Q11) consistently clustered together. Items “pain” (Q5), “felt nauseated” (Q9), “pain interference” (Q12) usually clustered together. At the day 5 follow-up, “short walk” (Q1), “in bed” (Q2), “need help” (Q3), “short of breath” (Q4), “felt weak” (Q7), and “been tired” (Q11) repeatedly clustered together, similar to their behavior at baseline. Slightly different from baseline, “pain” (Q5), “trouble sleeping” (Q6) and “pain interference” (Q12) usually clustered together. At day 10 follow-up, items “pain” (Q5), “trouble sleeping” (Q6) and “pain interference” (Q12) clustered together like they did at day 5. Departing from baseline and day 5, “short walk” (Q1), “in bed” (Q2), “need help” (Q3), “short of breath” (Q4) and “been tired” (Q11) clustered together. At all three intervals, “felt tense” (Q13) and “felt depressed” (Q14) consistently clustered together.
Full table
From baseline to day 10 follow-up, across all types of analyses, items associated with respiratory (shortness of breath) and physical functioning (walking ability, staying in a chair/bed, weakness, tiredness) clustered together with exception of weakness at day 10. At baseline, pain and pain interference clustered with nausea; whereas, at both follow-ups, they clustered with sleep.
Palliative radiotherapy can impact QoL in a variety of ways, which is reflected in changes documented by assessment tools (14). Due to the different characteristics of the various questionnaires used in previously conducted symptom cluster research in palliative oncology patients, it is difficult to make comparisons with the results from the present study. In a review of clusters reported in 32 observational studies, Dong et al. identified 4 consistent groups of symptoms in patients with advanced cancer: anxiety-depression, nausea-vomiting, nausea-appetite loss, and fatigue-dyspnea-drowsiness (4). Early symptom cluster research focused on the relationships between pain, fatigue and sleep disturbances in cancer patients (2). Mediation analyses in a study conducted by Beck et al. later indicated that pain both directly and indirectly (through sleep disturbance) impacted fatigue (15).
Using data from a face-valid symptom reporting form, Tsai et al. reported and termed 5 different symptom clusters: loss of energy (fatigue, weakness), poor intake (anorexia, taste alteration, dysphagia, constipation, dry mouth/thirsty), autonomic dysfunction (restlessness/heat, dizziness, insomnia, night sweats), aerodigestive impairment (nausea/vomiting, abdominal fullness, dyspnea) and pain complex (16). Chen et al. performed the same three statistical procedures (HCA, PCA, EFA) on Brief Pain Inventory (BPI) data collected in a similar sample of patients at 4-, 8- and 12-week follow-up intervals (17). The authors observed varying patterns of symptom cluster presentation over time in different subgroups (e.g., responders, non-responders); however, they noticed a frequent core cluster of symptoms consisting of worst pain, general activity, walking ability, normal work, and enjoyment of life. Khan et al. conducted PCA, HCA and EFA analyses on ESAS data collected at 1, 2-, 4-, 8- and 12-week following radiation in a similar patient population (18). At baseline, the PCA and HCA identified three clusters: (I) depression and anxiety; (II) fatigue, drowsiness, pain, and poor well-being; and (III) nausea, poor appetite, and dyspnea. Similarly, two clusters were observed with the EFA: (I) depression and anxiety; and (II) fatigue, drowsiness, pain, nausea, poor appetite, dyspnea, and poor well-being. At 1 week, the PCA and HCA identified three clusters: (I) pain, nausea and poor appetite; (II) fatigue, drowsiness, and dyspnea; and (III) depression, anxiety, and poor well-being. At 2 weeks, the PCA and HCA identified two clusters: (I) depression, anxiety, nausea, poor appetite, and poor well-being; and (II) fatigue, drowsiness, and pain. The same two clusters were observed with the EFA at 1- and 2-week: (I) depression, anxiety, nausea, and poor well-being; and (II) fatigue, drowsiness, pain, poor appetite, and dyspnea. Pain clustered with nausea at 1 week, and clustered with fatigue and drowsiness at baseline and 2 weeks. All patients in the present study were prescribed anti-emetics, and this may contribute to the difference observed. BPI and ESAS focus on interference with function, and symptoms respectively. In using the C15-PAL, we were able to study relationships between both symptoms and interference with function which are relevant to palliative patients. However, more investigation is required using this tool to validate the clusters identified.
In a secondary analysis conducted of the NCIC CTG SC.23 trial, McDonald et al. reported that 40% of patients experienced pain reduction and improved QoL at day 10 post-radiation using the EORTC QLQ-Bone Metastases Module (BM22) in addition to the C15-PAL (19). Another significant influence on pain during this shorter follow-up period is pain flare, which was reported in 40% of patients with bone metastases within 10 days post-radiotherapy (20). Patients in this particular analysis were also prescribed anti-emetics, as recommended by current guidelines, from which patients may experience side-effects such as headache or constipation in the follow-up period (5). Other limitations of the study include the large proportion of patients with bone metastases, and the short observation time. We were also unable to explore changes in symptom clustering with those who responded to radiation treatment versus those who did not.
Our results suggest dynamism of symptom clusters immediately following radiotherapy treatment in this patient population, which should be further explored in future symptom cluster research. Three separate analyses of data collected by a palliative QoL assessment tool in patients with advanced cancer undergoing radiation treatment were used. Respiratory and physical functions were found to frequently cluster together. Pain and pain interference clustered with nausea at baseline, and subsequently, with sleep at follow-up. Fluctuation of symptom clusters was observed in a short time frame following radiation.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional research ethics board of Sunnybrook Health Sciences Centre (No. 434-2013). All patients provided informed consent.
References
- Fan G, Filipczak L, Chow E. Symptom clusters in cancer patients: a review of the literature. Curr Oncol 2007;14:173-9. [Crossref] [PubMed]
- Dodd MJ, Miakowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum 2001;28:465-70. [PubMed]
- Bennion AE, Molassiotis A. Qualitative research into the symptom experiences of adult cancer patients after treatments: a systematic review and meta-analysis. Support Care Cancer 2013;21:9-25. [Crossref] [PubMed]
- Dong ST, Butow PN, Costa DS, et al. Symptom clusters in patients with advanced cancer: a systematic review of observational studies. J Pain Symptom Manage 2014;48:411-50. [Crossref] [PubMed]
- Wong E, Pulenzas N, Bedard G, et al. Ondansetron rapidly dissolving film for the prophylactic treatment of radiation-induced nausea and vomiting-a pilot study. Curr Oncol 2015;22:199-210. [Crossref] [PubMed]
- Groenvold M, Petersen MA, Aaronson NK, et al. The development of the EORTC QLQ-C15-PAL: A shortened questionnaire for cancer patients in palliative care. Eur J Cancer 2006;42:55-64. [Crossref] [PubMed]
- Kruskal JB. Nonmetric multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 1964;29:1-27. [Crossref]
- Kline P. The handbook of psychological testing. 2nd edition. London, New York: Routledge, 2000.
- DeVellis RF. Scale development: Theory and applications. 4th Edition. Los Angeles: Sage, 2012.
- Barsevick AM, Whitmer K, Nail LM, et al. Symptom cluster research: conceptual design, measurement, and analysis issues. J Pain Symptom Manage 2006;31:85-95. [Crossref] [PubMed]
- Kim HJ, McGuire DB, Tulman L, et al. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs 2005;28:270-82. [Crossref] [PubMed]
- Kim HJ, Abraham IL. Statistical approaches to modeling symptom clusters in cancer patients. Cancer Nurs 2008;31:E1-10. [Crossref] [PubMed]
- Skerman HM, Yates PM, Battistutta D. Multivariate methods to identify cancer-related symptoms clusters. Res Nurs Health 2009;32:345-60. [Crossref] [PubMed]
- McDonald R, Chow E, Rowbottom L, et al. Quality of life after palliative radiotherapy in bone metastases: A literature review. J Bone Oncol 2014;4:24-31. [Crossref] [PubMed]
- Beck SL, Dudley WN, Barsevick A. Pain, sleep disturbance, and fatigue in patients with cancer: using a mediation model to test a symptom cluster. Oncol Nurs Forum 2005;32:542. [Crossref] [PubMed]
- Tsai JS, Wu CH, Chiu TY, et al. Significance of symptom clustering in palliative care of advanced cancer patients. J Pain Symptom Manage 2010;39:655-62. [Crossref] [PubMed]
- Chen E, Khan L, Zhang L, et al. Symptom clusters in patients with bone metastases--a reanalysis comparing different statistical methods. Support Care Cancer 2012;20:2811-20. [Crossref] [PubMed]
- Khan L, Cramarossa G, Chen E, et al. Symptom clusters using the Edmonton Symptom Assessment System in patients with bone metastases: a reanalysis comparing different statistical methods. World J Oncol 2012;3:23-32. [PubMed]
- McDonald R, Ding K, Brundage M, et al. Effect of Radiotherapy on Painful Bone Metastases: A Secondary Analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol 2017;3:953-9. [Crossref] [PubMed]
- Hird A, Chow E, Zhang L, et al. Determining in the incidence of pain flare following palliative radiothrapy for symptomatic bone metastases: results from three Canadian cancer centers. Int J Radiat Oncol Biol Phys 2009;75:193-7. [Crossref] [PubMed]


