Palliative concurrent chemoradiotherapy in locally advanced and metastatic esophageal cancer patients with dysphagia
Introduction
In the United States, an estimated 17,990 cases of esophageal cancer will be diagnosed in 2013, and 15,210 deaths are expected from the disease (1). Worldwide, an estimated 482,300 new esophageal cancer cases and 406,800 deaths occurred in 2008 (2). Regardless of histology, approximately 50 to 60 percent of patients with esophageal cancer present with incurable locally advanced or metastatic disease (3).
Dysphagia is the most common and serious symptom in patients with unresectable, metastatic esophageal cancer, it severely affects the patient’s quality of life and necessitates nutritional support, so long-term relief of dysphagia is one of the most important issues in their daily life (4).
Several management options have been developed to palliate malignant dysphagia. These include endoluminal stenting, surgery, external beam radiation, brachytherapy, chemotherapy, chemoradiotherapy, laser treatment, photodynamic therapy or ablation using injection of alcohol or chemotherapeutic agents (5,6).
In a 2013 guideline, the American Society for Gastrointestinal Endoscopy recommended esophageal stenting as the preferred method for palliation of dysphagia and fistulas in patients with esophageal cancer (7).
Of the multiple treatment options, chemoradiotherapy had been reported to be effective for the palliation of dysphagia through tumor regression in advanced, incurable esophageal cancer but it takes 2-4 weeks to relieve obstruction, whereas immediate relief can be achieved with stent placement or feeding tube (8-12).
The aim of this prospective study was to evaluate the efficacy and toxicity of palliative chemoradiotherapy in locally advanced and metastatic esophageal cancer as regard improvement of dysphagia, primary tumor response and survival time.
Patients and methods
This prospective study was conducted at the Clinical Oncology and Nuclear Medicine Department, Mansoura university Hospital, Egypt between August 2010-July 2012 and included twenty eight patients with locally advanced and metastatic esophageal carcinoma, 2 patients died before the start of chemoradiotherapy and another one developed severe chest infection, so the 3 patients were excluded from the study.
Eligibility also required that subjects be ≤75 years of age with an Eastern Cooperative Oncology Group performance status 0 to 2, WBC count ≥3,000/µL, platelet count ≥100,000/µL, AST and ALT levels within three times the normal upper limit, serum bilirubin level ≤2.0 mg/dL, creatinine level ≤1.5 mg/dL, creatinine clearance ≥50 mL/min, normal electrocardiogram, and life expectancy ≥8 weeks. Patients with serious complications were excluded from the study. Patients with esophagobronchial fistulas were also excluded from the study.
Pre-study evaluation included barium esophagography, panendoscopy including laryngeal, pharyngeal and esophagoscopy, and neck, chest, and abdominal computed tomography (CT) scans. Dysphagia was measured at the beginning and completion of treatment and at monthly intervals until death, using a 5-point dysphagia score (3,13-15).
Treatment schedule
Radiation treatment (6 MV) was administered for 4.5 weeks (5 days/week) at 1.8 Gy/day with a total radiation dose of 40 Gy/22 fractions, concomitantly with chemotherapy. The targeted area for irradiation included only the primary tumor with a 3 cm superior and inferior margin and a 2 cm lateral margin. Irradiation was applied using anterior and posterior opposed fields. Chemotherapy regimens consisted of cisplatin 70 mg/m2 infusion on day 1, plus continuous infusion of 5-fluorouracil at 700 mg/m2 per day from day 1 to day 4.
For patients who showed an objective response to treatment, additional courses of chemotherapy were administered, which consisted of the same regimen of protracted infusional 5-FU 800 mg/m2/day on days 1-5 and a 2 h infusion of cisplatin 80 mg/m2/day on day 1. Treatment was repeated every 4 weeks until disease progression, development of unacceptable toxicity or the patient’s refusal to continue. The doses of 5-FU and cisplatin were reduced to 75% if grade 4 leukocytopenia, thrombocytopenia, diarrhea, grade 3 mucositis, or esophagitis was observed during the previous course.
Response and toxicity evaluation
Haematological and non haematological toxicities were recorded according to the Common Terminology Criteria for Adverse Events v3.0 (CTCAE).Toxicity was assessed on a weekly basis during chemoradiotherapy. The grade of dysphagia was determined by the dysphagia score as previously described in Table 1. Medical management included antacids for esophagitis, antiemetics for nausea and vomiting. Occasionally, patients may need intravenous hydration due to dehydration from poor oral intake, and when necessary, nutritional support was provided by intravenous hyperalimentation. Improvement of dysphagia was defined as a decrease of at least 1 point in dysphagia score.

Full Table
Tumor response was evaluated using computed tomography scan and endoscopy 8 weeks after starting treatment. Response of the primary tumor was evaluated by the criteria of the Japan Esophageal Society (16-18). Complete response (CR) of the primary lesion is judged, using endoscopy, with the fulfillment of all of the following conditions: (I) disappearance of all endoscopic findings that suggest the presence of tumor, such as irregular erosive lesions, ulcerative lesions or obvious elevated lesions; (II) no histologic findings of malignant cells by endoscopic biopsy from the area where the primary tumor had been; (III) the entire esophagus can be observed by endoscopy; and (IV) no findings of active esophagitis by endoscopy. Progressive disease (PD) of the primary lesion means distinct tumor growth or progression in esophageal stenosis during treatment. Incomplete response/stable disease (IR/SD) means that the response of the primary lesion does not meet the conditions for CR or PD.
Statistical analysis
The statistical analysis of data was done using SPSS program for windows version 17. The descriptive data was done in the form of median for quantitative data, frequency and proportion for qualitative data. Overall (OS) and progression free survival (PFS) survival were determined using Kaplan Meier method to provide the median value and 95% CI. Survival curves were calculated from life tables.
The primary end point of the study was efficacy of chemoradiotherapy regimen as regard dysphagia improvement, response of the primary tumor and toxicity, while secondary end points were overall (OS) and progression free survival (PFS).
Results
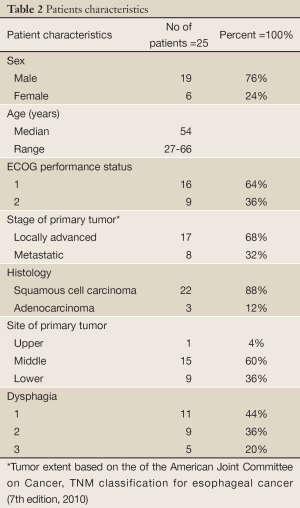
Characteristics of the 25 patients are listed in Table 2. Dysphagia improved in 18 (72%) of the 25 patients. The median duration of dysphagia improvement was 5 months after treatment in these patients. The proportion of dysphagia 1, 2, 3 before treatment was 11 (44%), 9 (36%) and 5 (20%), respectively. While after treatment, the proportion of dysphagia 0, 1, 2 and 3 was 9 (36%), 9 (36%), 5 (20%) and 2(8%), respectively.
Full Table
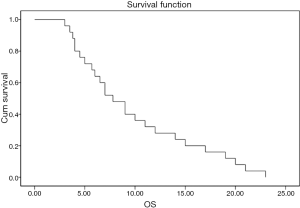
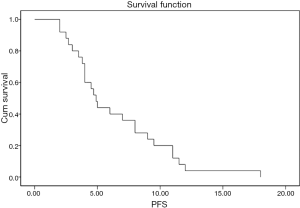
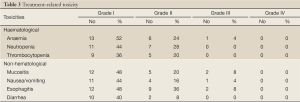
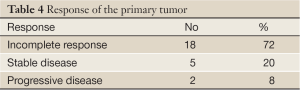
The grades of toxicity during the treatment course are summarized in Table 3. The results of the overall response are summarised in Table 4 . Of the 25 eligible patients, 18 (72%) achieved incomplete response PR, five patients (20%) showed stable disease SD, and two (8%) had progressive PD. The median overall and the progression free survival times were 7 and 4 months, respectively (Figures 1,2). All patients completed the planned chemoradiotherapy course.
Full Table
Full Table
Discussion
Dysphagia from inoperable esophageal cancer is a common and complex management problem, and there is no consensus on the ideal treatment approach (19). Dysphagia may progress rapidly to a stage where patients are unable to swallow liquids and saliva, making them prone to nutritional compromise and aspiration pneumonia. So, long-term palliation of dysphagia is an important goal of therapy (3). Several management options have been developed to palliate malignant dysphagia (5,6).
Although external-beam radiation with or without chemotherapy takes at least 2 weeks to produce palliation, its effect is more durable than that provided by the other palliative modalities because external-beam radiation treats the problem (the gross tumor mass), not just the symptom (20).
Esophageal dilatation with either the-scope balloon or wire-guided polyvinyl bougies can provide temporary relief of dysphagia until more definitive treatment can be accomplished. Most malignant strictures can be safely dilated to 16 or 17 mm in several sessions (21). Esophageal dilation is also associated with a small risk of perforation, especially if performed by blind Maloney dilation during radiotherapy (22-25).
Our study, assessed concomitant chemoradiation for patients with locally advanced and metastatic esophageal carcinoma having dysphagia. Dysphagia improved in 72% of patients with median duration of improvement of 5 months.
A phase I/II trial from Canada prospectively treated 22 patients with dysphagia from advanced incurable esophageal cancer with palliative radiation (30 Gy in 10 fractions) with a concurrent single course of chemotherapy (5-FU, 1,000 mg/m2, days 1-4 and mitomycin-C 10 mg/m2, day 1, showed that the combined regimen was well tolerated, and close to 70% patients achieved a CR (i.e., no difficulty on swallowing) with a median time to normalization of swallowing of 5 weeks (18). This high remarkable percent of complete response may be attributed to different patients characteristics, a higher dosage of 5-FU than ours and the use of different doublet with 5-FU.
Our results are comparable to that of a retrospective study used palliative chemoradiotherapy in stage IVB esophageal cancer patients with dysphagia. The treatment consisted of two courses of chemotherapy (5-fluorouracil and cisplatin) and concurrent irradiation of 40 Gy in 20 fractions to the esophageal primary tumor. Dysphagia score improved in 75% of the patients. Disease control rate of the primary lesion was 95%, with 12 patients (30%) achieved a complete response. The overall response rate was 55%. The median survival was 308 days, and the 1-year-survival rate was 45.0%. The median progression-free survival was 139 days (26).
Also, our results are in accordance with most studies evaluating palliative chemoradiotherapy in advanced and metastatic esophageal carcinoma, as regard the dysphagia improvement rate, median overall survival, predominance of sqamous cell carcinoma and treatment toxicity, while our median age is younger by about one decade than most of these studies, which arouses attention to study median age in a large sample (8,11,12,18,25,26).
In a study by Wong et al., a total of 36 consecutive patients (33 male, mean ± SD age 63.2±9.5 years) with T4 disease (81%) with or without cervical nodal metastasis (50%) received CRT, while 36 patients treated with endoscopic stenting alone were recruited as controls. Both groups were comparable in demographics, pretreatment dysphagia score comorbidities, and tumor characteristics. CRT was completed in 32 patients (89%). There was no treatment-related mortality. Tumor volume was greatly reduced after CRT in 19 patients. Four patients (11%) received salvage esophagectomy 9 to 42 months after CRT. Compared with the stenting group, CRT statistically significantly improved 5-year survival (15% vs. 0%, P=0.01), median survival (10.8 vs. 4.0 months, P<0.005), and need for stenting (22% vs. 100%, P=005) (27).
These encouraging results of CRT versus stenting need further large randomized prospective trials ensuring these results.
Conclusions
Palliative chemoradiotherapy using 5-FU plus cisplatin combined with concurrent 40 Gy/4.5 weeks/22 fractions effectively improved dysphagia in locally advanced and metastatic esophageal cancer patients with acceptable toxicity and favorable survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Javle M, Ailawadhi S, Yang GY, et al. Palliation of malignant dysphagia in esophageal cancer: a literature-based review. J Support Oncol 2006;4:365-73, 379. [PubMed]
- Watt E, Whyte F. The experience of dysphagia and its effect on the quality of life of patients with oesophageal cancer. Eur J Cancer Care (Engl) 2003;12:183-93. [PubMed]
- Weigel TL, Frumiento C, Gaumintz E. Endoluminal palliation for dysphagia secondary to esophageal carcinoma. Surg Clin North Am 2002;82:747-61. [PubMed]
- Siersema PD, Dees J, van Blankenstein M. Palliation of malignant dysphagia from oesophageal cancer. Rotterdam Oesophageal Tumor Study Group. Scand J Gastroenterol Suppl 1998;225:75-84. [PubMed]
- ASGE Standards of Practice Committee, Evans JA, Early DS, et al. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328-34. [PubMed]
- Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260-5. [PubMed]
- Knyrim K, Wagner HJ, Bethge N, et al. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med 1993;329:1302-7. [PubMed]
- Raijman I, Siddique I, Ajani J, et al. Palliation of malignant dysphagia and fistulae with coated expandable metal stents: experience with 101 patients. Gastrointest Endosc 1998;48:172-9. [PubMed]
- Burmeister BH, Walpole ET, Burmeister EA, et al. Feasibility of chemoradiation therapy with protracted infusion of 5-fluorouracil for esophageal cancer patients not suitable for cisplatin. Int J Clin Oncol 2005;10:256-61. [PubMed]
- Cho SH, Shim HJ, Lee SR, et al. Concurrent chemoradiotherapy with S-1 and cisplatin in advanced esophageal cancer. Dis Esophagus 2008;21:697-703. [PubMed]
- Gaspar LE, Winter K, Kocha WI, et al. Swallowing function and weight change observed in a phase I/II study of external-beam radiation, brachytherapy and concurrent chemotherapy in localized cancer of the esophagus (RTOG 9207). Cancer J 2001;7:388-94. [PubMed]
- Blazeby JM, Vickery CW. Quality of life in patients with cancers of the upper gastrointestinal tract. Expert Rev Anticancer Ther 2001;1:269-76. [PubMed]
- Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg 2001;127:870-6. [PubMed]
- Diseases JSfE. Guidelines for the Clinical and Pathological Studies on Carcinoma of the Esophagus (in Japanese). 9th edn. Tokyo: Kanehara Shuppan 1999:59-79.
- Takubo K, Makuuchi H, Fujita H, et al. Japanese Classification of Esophageal Cancer, tenth edition: part 1. Esophagus 2009;6:1-25.
- Hayter CR, Huff-Winters C, Paszat L, et al. A prospective trial of short-course radiotherapy plus chemotherapy for palliation of dysphagia from advanced esophageal cancer. Radiother Oncol 2000;56:329-33. [PubMed]
- Hanna WC, Sudarshan M, Roberge D, et al. What is the optimal management of dysphagia in metastatic esophageal cancer? Curr Oncol 2012;19:e60-6. [PubMed]
- Posner MC, Minsky BD, Ilson DH. Cancer of the Esophagus. Section 2; Devita, Hellman & Rosenberg’s Cancer: Principles & Practice of Oncology, 8th Edition 2008;993-1036.
- Boyce HW Jr. Palliation of Dysphagia of Esophageal Cancer by Endoscopic Lumen Restoration Techniques. Cancer Control 1999;6:73-83. [PubMed]
- Heit HA, Johnson LF, Siegel SR, et al. Palliative dilation for dysphagia in esophageal carcinoma. Ann Intern Med 1978;89:629-31. [PubMed]
- Cassidy DE, Nord HJ, Boyce HW. Management of malignant esophageal strictures: Role of esophageal dilation and peroral prosthesis. Am J Gastroenterol 1981;75:173.
- Lundell L, Leth R, Lind T, et al. Palliative endoscopic dilatation in carcinoma of the esophagus and esophagogastric junction. Acta Chir Scand 1989;155:179-84. [PubMed]
- Hernandez LV, Jacobson JW, Harris MS. Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures. Gastrointest Endosc 2000;51:460-2. [PubMed]
- Ikeda E, Kojima T, Kaneko K, et al. Efficacy of concurrent chemoradiotherapy as a palliative treatment in stage IVB esophageal cancer patients with dysphagia. Jpn J Clin Oncol 2011;41:964-72. [PubMed]
- Wong SK, Chiu PW, Leung SF, et al. Concurrent chemoradiotherapy or endoscopic stenting for advanced squamous cell carcinoma of esophagus: a case-control study. Ann Surg Oncol 2008;15:576-82. [PubMed]