Treatment of brain metastases in non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations: the role of EGFR tyrosine kinase inhibitors
The brain is one of the most common sites for lung adenocarcinoma metastasis, which are found in almost 20-30% of patients with the disease (1). These patients have poor median survival, and more effective therapies are urgently required. Since traditional chemotherapy is less effective against metastatic brain tumors, radiotherapy remains the main therapeutic or palliative option for inoperable central nervous system (CNS) disease. Radiotherapy supplemented with steroids has yielded responses rates of 50-75% for intracranial lesions, providing rapid attenuation of neurologic symptoms and improvement of performance status (2). However, brain metastases still herald a poor prognosis with a median survival of less than six months (3). Age, performance status, control of primary tumor, extend of extracranial disease, number of brain metastases, aggressive treatment modalities like surgery or radiosurgery, but also biomarkers such as expression levels of vascular enthothelial growth factor (VEGF), cyclooxygenase-2, epidermal growth factor receptor (EGFR) overexpression, and EGFR mutations have been explored as prognostic factors for patients with CNS metastases (4).
EGFR is a transmembrane protein with cytoplasmic kinase activity that can transduce growth factor signaling from the extracellular domain to the cell. Non-small cell lung cancer (NSCLC) patients with activating somatic mutations in the region of the EGFR gene that encodes the tyrosine kinase domain (such as mutations in exons 19 and 21) are highly responsive to EGFR-tyrosine kinase inhibitors (TKIs) and, as illustrated by individual trials and meta-analyses, EGFR TKIs can significantly improve progression-free survival compared with standard chemotherapy (5-7). It is also known that certain clinical or demographic characteristics, such as being Asian, female, a never smoker or adenocarcinoma or bronchioloalveolar carcinoma (BAC) tumor histology, are associated with a higher probability of response to EGFR TKIs (8).
The Journal of Clinical Oncology has featured recently the results of a multi-institutional phase II study of the EGFR inhibitor erlotinib with whole-brain radiation therapy (WBRT) for patients with brain metastases from a NSCLC primary disease (9). Forty NSCLC patients with brain metastases, regardless of EGFR status, received erlotinib monotherapy for one week, then concurrently with WBRT, followed by maintenance, a treatment which was feasible and tolerable and produced longer overall survival compared with historical controls, with particular benefit evident for patients with EGFR mutations (9). Overall response rate was 86% and at a median follow up of more than two years, median survival was 11.8 months, almost double the 6 months the study was designed to demonstrate (9). These results are impressive but require interpretation in the context of information which has become available since the enrollment of this trial began (7). Furthermore, the results of a recently published study indicate that patients with EGFR mutations may have prolonged survival after diagnosis of brain metastases, independent of treatment strategy (10). In recent years, there has been growing interest in the potential CNS activity of EGFR TKIs alone in NSCLC patients with EGFR activating mutations; the idea that erlotinib or gefitinib may delay or obviate the need for brain radiation is appealing. However, the role of EGFR TKIs in the treatment of brain metastases remains unclear since data on the use of erlotinib or gefitinib are available from retrospective and non-randomized studies with a limited number of patients (Table 1).
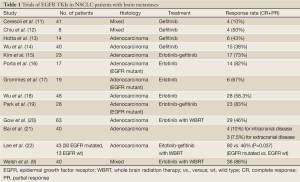
Full Table
In the study from Welsh et al. patients were enrolled regardless of EGFR status. Among 17 patients, for whom EGFR status was tested by DNA sequencing, median survival was 9.3 months for those with (WT) type EGFR and 19.1 months for those with EGFR mutations but this apparent difference was not statistically significant, highlighting the preferential activity of erlotinib in EGFR mutated tumors (9). However, more than 50% of patients who were tested for EGFR mutations had tumors with an EGFR activating mutation, a much higher percentage than what is normally expected from a random sample of NSCLC patients. It is possible that selection bias in this trial could explain this high percentage, since the trial demographics included younger age, a higher percentage of women and a higher percentage of never smokers than what is typical for patients with metastatic NSCLC (9). Therefore, the conclusions warrant further exploration before we can consider them indicative of EGFR mutated tumors having a CNS tropism.
In vitro studies have demonstrated the radiosensitivity of lung cancer cells with mutant EGFR (23). However, in the present study the authors emphasize in vitro data which demonstrate that EGFR TKIs can radiosensitize EGFR wild type tumors (24). Though overexpression of EGFR has been examined as a mechanism of resistance to radiotherapy, EGFR blockade has not been beneficial in tumors such as glioblastoma multiforme with increased expression of EGFR (25). Welsh et al. comment on the benefit of erlotinib and WBRT in EGFR WT and unknown status patients. However, this benefit still remains unclear without definite knowledge of the mutational status of the unknown group. It should also be borne in mind in this study the number of EGFR mutants was much higher than one would expect.
In conclusion, the trial conducted by Welsh et al. shows that the combination of erlotinib and WBRT is feasible, safe and effective. Though these results are provoking, they should be interpreted cautiously due to the small number of patients, lack of randomization and changes in the standard of care for patients with EGFR mutated tumors. Since erlotinib or gefitinib can be effective monotherapy for treatment of patients with EGFR activating mutations and brain metastases—as suggested by recent papers—the combination of EGFR TKIs and WBRT may be a reasonable option for the highly symptomatic subgroup of patients for whom it could be detrimental to wait for response to EGFR TKIs, or for those with numerous brain lesions that cannot be treated with stereotactic radiosurgery. Larger prospective randomized clinical trials that will confirm the role of EGFR TKIs in the response of brain metastases, alone or in combination with WBRT are awaited.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 1988;6:1474-80. [PubMed]
- Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005;23:6207-19. [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [PubMed]
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR Inhibitor in Non-Small Cell Lung Cancer on Progression-Free and Overall Survival: A Meta-Analysis. J Natl Cancer Inst 2013;105:595-605. [PubMed]
- Tsao AS, Tang XM, Sabloff B, et al. Clinicopathologic characteristics of the EGFR gene mutation in non-small cell lung cancer. J Thorac Oncol 2006;1:231-9. [PubMed]
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. [PubMed]
- Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol 2010;12:1193-9. [PubMed]
- Ceresoli GL, Cappuzzo F, Gregorc V, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol 2004;15:1042-7. [PubMed]
- Chiu CH, Tsai CM, Chen YM, et al. Gefitinib is active in patients with brain metastases from non-small cell lung cancer and response is related to skin toxicity. Lung Cancer 2005;47:129-38. [PubMed]
- Hotta K, Kiura K, Ueoka H, et al. Effect of gefitinib (‘Iressa’, ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer 2004;46:255-61. [PubMed]
- Wu C, Li YL, Wang ZM, et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 2007;57:359-64. [PubMed]
- Kim JE, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 2009;65:351-4. [PubMed]
- Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J 2011;37:624-31. [PubMed]
- Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 2011;13:1364-9. [PubMed]
- Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol 2013;24:993-9. [PubMed]
- Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556-60. [PubMed]
- Gow CH, Chien CR, Chang YL, et al. Radiotherapy in lung adenocarcinoma with brain metastases: effects of activating epidermal growth factor receptor mutations on clinical response. Clin Cancer Res 2008;14:162-8. [PubMed]
- Bai H, Han B. The effectiveness of erlotinib against brain metastases in non-small cell lung cancer patients. Am J Clin Oncol 2013;36:110-5. [PubMed]
- Lee HL, Chung TS, Ting LL, et al. EGFR mutations are associated with favorable intracranial response and progression-free survival following brain irradiation in non-small cell lung cancer patients with brain metastases. Radiat Oncol 2012;7:181. [PubMed]
- Das AK, Sato M, Story MD, et al. Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res 2006;66:9601-8. [PubMed]
- Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res 2005;65:3328-35. [PubMed]
- Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol 2008;26:5603-9. [PubMed]


