
Immunotherapy and radiotherapy for metastatic cancers
Introduction
Immune checkpoint inhibitors (ICIs) have dramatically altered the landscape of treatment for metastatic solid tumors. By removing the “brakes” on the immune system, agents that target the inhibitory CTLA-4 and PD-1 receptors invigorate anti-tumor immune responses. Numerous landmark trials demonstrate the effectiveness of ICIs across tumor histologies, including malignant melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), urothelial carcinoma, head and neck squamous cell carcinoma (HNSCC), gastric adenocarcinoma, mismatch-repair-deficient solid tumors, Hodgkin’s lymphoma and Merkel cell carcinoma, where treatment with a CTLA-4, PD-1, or PD-L1 inhibitor are FDA-approved (1-9). Particularly notable among successful landmark phase 3 immunotherapy trials is a population of long-term responders along with a relatively larger population of non-responders who derive minimal, if any, benefit from therapy (10). This potential for long-term benefit, which has now extended beyond a decade in melanoma patients, has ignited intense interest in combination treatment strategies, with the goal of maximizing the number of these long-term responders (11). Early successes include the combination of PD-1 and CTLA-4 inhibition in melanoma and renal cell carcinoma (12,13), as well as PD-1 inhibition and chemotherapy in patients with metastatic non-small cell lung cancer (14).
Take home points:
- Palliative radiotherapy and more recently, immune checkpoint therapy occupy a prominent role in the treatment of metastatic disease. The combination of these treatments appears to be generally well tolerated, although practitioners need to be vigilant to identify and treat immune mediated toxicities. Potential impacts on radionecrosis in patients receiving stereotactic radiosurgery and immune checkpoint inhibitors must be balanced against favorable clinical outcomes now observed with the combination of the two treatments.
- Emerging data suggest that the addition of radiation to patients with limited progression on immunotherapy may be an effective treatment strategy to allow select patients to continue to benefit from immune checkpoint blockade.
- Preclinical studies and clinical data have suggested that the addition of radiotherapy to immune checkpoint blockade could improve systemic response rates—this strategy is being evaluated in prospective clinical trials.
Given that the majority of solid tumor patients will receive radiation therapy over the course of their illness (15), the combination of radiotherapy and immunotherapy for metastatic cancers is particularly appealing. In preclinical models, radiotherapy combined with ICI both enhances the local impact of radiation and the systemic effects of immunotherapy. The immunogenic effects of radiation include enhanced STING (stimulator of interferon genes) pathway activation, antigen presentation, T-cell activation and trafficking that may complement the immune effects of both CTLA-4 and PD-1 pathway inhibition (16). In particular, radiotherapy gives rise to increased uptake of exogenous DNA into the cytoplasm, upregulating interferon production through the cGAS-STING pathway, which in turn helps facilitate a mounted adaptive immune response (17). There is significant interest in understanding and harnessing rare abscopal responses that occur outside of the treatment field following targeted radiation that are likely immune mediated. However, when administered in combination with ICI, it becomes challenging to distinguish between the abscopal effect, enhanced activity of ICI, and delayed and atypical patterns of response that can be observed after both CTLA-4 and PD-1 monotherapy (18-22). Thus, prospective and ideally, randomized trials incorporating ICI and radiation are needed to demonstrate benefit.
Additionally, synergy between radiation and immunotherapy is a double-edged sword, potentially increasing the risk of either radiotherapy-mediated or immune-mediated toxicity. Inhibiting important immunologic checkpoints could increase immune cell-mediated normal tissue damage at the irradiated site. Conversely, by provoking normal tissue injury or acting through immunologic pathways, radiotherapy could worsen immune-related adverse events (ir-AEs) observed with ICIs.
In this review, we discuss the literature and present the current clinical data on the safety of combining ICIs with radiotherapy in patients with metastatic cancers, focusing on associations with the timing of radiation administration, as well as on two toxicities of particular concern in metastatic patients receiving either brain or lung directed radiation: radionecrosis of the brain and pneumonitis. Finally, we examine the potential benefit of radiation/immunotherapy combinations on both local and systemic responses, and look towards the future to explore how to maximize benefit.
Toxicity
Overall rates of toxicity
One of the potential advantages of immunotherapy as compared to cytotoxic chemotherapy is the side effect profile which is distinct and often better tolerated. Immunologic toxicities associated with ICI vary by class of agent and include fatigue, dermatitis, myalgias, thyroid dysfunction, colitis and pneumonitis (23). However, although these immunologic toxicities are low grade in many patients, they can be severe or even fatal. Prompt recognition and treatment is required to minimize the risk of serious toxicity (24). Thus, identifying any heightened risks associated with the combination of radiation and immunotherapy is paramount.
Only recently have the first prospective studies examining the combination of radiotherapy and immunotherapy in the setting of metastatic disease been reported (Table 1). In these reports, grade 3–5 toxicities have ranged from 14–34% in trials using CTLA-4 inhibitors and 5–10% in patients receiving PD-1 inhibitors, generally consistent with the side effect profile expected with immune checkpoint therapy alone. Furthermore, there were few serious side effects attributed to the radiation component of treatment (18,25-30). However, none of these aforementioned studies randomized patients between ICI alone and ICI with radiotherapy, limiting the conclusions that can be drawn. More recently however, Theelen et al. and McBride et al. presented preliminary data at ASCO 2018 from two phase II randomized trials exploring the use of PD-1 inhibition with or without hypofractionated radiotherapy in patients with metastatic NSCLC and metastatic HNSCC, respectively. Theelen et al. demonstrated a grade 3+ toxicity rate of 22% in the control arm vs. 17% in the experimental arm (31) while McBride et al. demonstrated grade 3+ toxicities of 15% and 11% in the control and experimental arms, respectively (32). Additionally, although the phase 3 randomized PACIFIC study included locally advanced stage 3 NSCLC patients and not patients with metastatic disease, it was reassuring that grade 3+ toxicities were relatively balanced following definitive chemoradiation between the ICI and control groups (33).
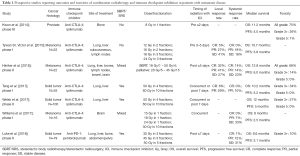
Full table
Thus, the combination of ICI and radiotherapy appears to be generally safe in most patients. It should be noted that grade 3–4 adverse events were more common in two trials conducted at a single institution in which ablative doses of radiotherapy (60 Gy delivered over 10 fractions, 50 Gy delivered over 4 fractions) were combined with the CTLA-4 inhibitor ipilimumab. Although these patients received ipilimumab at a dose of 3 mg/kg (approved for the treatment of metastatic melanoma patients), a grade 3–4 adverse event rate of 30% was more comparable to historical rates associated with ipilimumab dosed at 10 mg/kg (27). Reassuringly, these increased severe toxicity rates were not observed in other prospective trials combining radiation with ipilimumab, nor in the recently published prospective study by Luke et al., combining relatively high dose stereotactic body radiotherapy (SBRT) with the PD-1 inhibitor pembrolizumab where grade 3–4 toxicities were observed in 10% of patients (30).
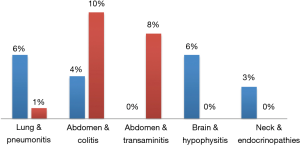
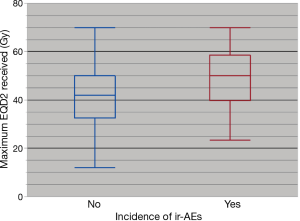
Although prospective data is limited, especially in regards to combinations with PD-1 inhibitors and radiation dose/fractionation regimens most commonly used in the setting of palliative treatment, there has been an expanding number of retrospective studies evaluating outcomes (Table 2) (34-54). Reassuringly, in these studies, grade 3 or higher toxicities have ranged from 8–41% in patients treated with CTLA-4 inhibitors and 5–13% with PD-1 inhibitors, again consistent with the historical rates of adverse events with ICI alone. In a systematic review of studies combining SRS and ICIs, the authors found that although the literature was limited, concurrent therapy in the treatment of brain mets appeared to be safe overall (55). In our recently published series, we found that overall, rates of all grade (35%) and grade 3+ toxicities (8%) were consistent with historical controls (43). We did not note any correlation between the sites of radiotherapy in increasing the rate of an associated adverse event (Figure 1) (43). Interestingly, there was an association between any grade ir-AEs and biologically effective radiation dose (BED) (P=0.01) as shown in Figure 2 (43). However, there was no association between BED and severe toxicity, and statistical significance was lost when evaluating only patients who received radiotherapy and a PD-1 inhibitor as opposed to a CTLA-4 inhibitor (43), similar to the findings in Luke et al.
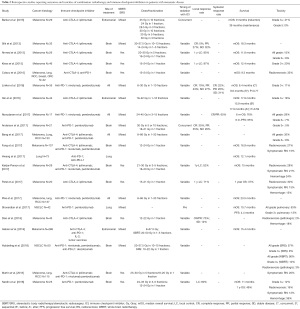
Full table
Questions regarding timing of therapy and development of toxicity
The relevance of timing, sequencing and interval between radiation and ICI is an increasingly common practical question for radiation and medical oncologists alike. It should be noted that in the prospective clinical trials published to date, radiotherapy was delivered concurrently or within 7 days of the administration of ICIs (18,25-32) with the exception of the PACIFIC trial, where patients were randomized between 1–42 days following chemoradiotherapy (33).
Several of the retrospective studies summarized in Table 2 investigated whether the sequencing (radiation before vs. after) or temporal proximity of radiation to ICI impacted overall rates of toxicity. With regards to the sequencing of therapies, both our group as well as Qin et al. did not identify a significant difference in the rates of ir-AEs depending on the sequencing of the two therapies (40,43). In contrast, there may be an association between the temporal proximity of the two treatments and overall toxicity rates. Kiess et al. evaluated patients with advanced melanoma treated with a CTLA-4 inhibitor and stereotactic radiosurgery (SRS), and demonstrated a higher rate of grade 3–4 toxicity when SRS was given “concurrently”, defined by the delivery of SRS within 1 month of the ICI (47% vs. 16%) (37). We identified a trend towards higher all grade ir-AEs when radiation was delivered within 2 weeks of the receipt of an ICI (39% vs. 23%, P=0.06), but there were no differences noted in the rate of grade 3–4 toxicities (43). It remains unproven whether radiation given in close sequence to ICI increases the rates of toxicity compared to radiation given concurrently. However, given the prolonged half-life and mechanism of action of these drugs, this seems unlikely. Ongoing and future prospective studies investigating the timing of radiotherapy in relation to ICI will help better establish whether there is any association between toxicity and either the sequencing and/or interval between treatments (56).
One of the most important limitations to identifying associations between combination radiation/ICI treatment and toxicity is that both immune and radiation-induced toxicities can manifest late months, or even years in the case of radiotherapy. Thus, long-term follow up of prospective clinical trials will be important to further investigate any potential increase in toxicity rates. In the meantime, vigilance is needed to identify toxicities with later onset. Multidisciplinary communication and collaboration is essential, especially in cases where patients have discontinued ICI treatment and may be receiving radiation monotherapy when they develop ir-AEs, or to help identify previous radiation treatment fields when toxicity develops after starting ICI. Particularly relevant to the latter are reports of recall ir-AEs that are localized to the radiation treatment field (57), which can only be recognized if the details of previous radiation treatment are known.
Brain radionecrosis
Although there is evidence that ICI monotherapy is efficacious in the brain (58,59), most patients with intracranial metastases are considered for radiotherapy, especially for histologies other than melanoma, where response rates to ICI are more limited. Thus, an increasing number of patients are treated with the combination of brain-directed radiotherapy and ICI. More specifically, the use of SRS for patients with limited numbers of brain metastases has continued to increase; thus, many of these patients will receive SRS in the setting of ICI. While it can be challenging to differentiate radionecrosis from progression or pseudoprogression, several retrospective studies have estimated that following SRS treatment, radiographic radionecrosis rates range from 16–33% (38,44,46,47,54), while symptomatic radionecrosis rates range from 12–21% (44,46,47,53,54). To help answer the question of whether the addition of ICIs increases the risk of radionecrosis, Martin et al. and Kaidar-Person et al. compared rates among patients who were treated with SRS and those who received both SRS and ICIs. Martin et al. studied 480 patients who received SRS in a mixed population (melanoma, NSCLC, RCC). They found that patients who received a CTLA-4 or PD-1 inhibitor had higher rates of symptomatic radionecrosis than those who received SRS alone (20% vs. 7%, HR 2.56, P=0.004) (53). Patients with metastatic melanoma appeared to be more susceptible to increased rates of symptomatic radionecrosis (HR 4.70, P=0.01) (53). Kaidar-Person et al. also demonstrated increased symptomatic radionecrosis rates of 21% compared to 0% in patients who did not receive an ICI (46). Comparing the impact of CTLA-4 inhibitors and PD-1 inhibitors, the study by Martin et al. demonstrated both classes of ICIs may numerically increase rates of radionecrosis, although the association with PD-1 inhibition was not statistically significant (HR 3.57, P=0.06) (53). Of note, an increasing number of studies demonstrate favorable survival in patients treated with the combination of SRS and brain metastases as compared to historical controls; this may impact the detection of radionecrosis in ways that are challenging to control for in retrospective studies.
Pneumonitis
In comparison to cytotoxic chemotherapy and CTLA-4 inhibitors, PD-1 inhibitors are associated with increased rates of pneumonitis. In a meta-analysis of 16 phase II/III studies, Wu et al. found the incidence of all grade pneumonitis was 2.9% and 1.5% for grade 3+ pneumonitis (60). Radiation-related pneumonitis is associated with a high dose of radiation to the lungs, and thus special attention is paid to the incidence of this toxicity when combining both treatments. When pneumonitis does develop in the setting of ICI in a patient previously treated with thoracic radiotherapy, it can be challenging to determine whether this is radiation or ICI related; this may have therapeutic implications if it prompts discontinuation of ICI therapy. Until better tools are available, evaluating the anatomical location of pneumonitis (radiation pneumonitis is generally localized to the radiation treatment field when detected in early stages, immune related pneumonitis is more diffuse) (61) and timing (radiation pneumonitis typically develops 2–9 months after radiotherapy (62) and immune related pneumonitis generally occurs between 0–19 months) (63) are generally the most helpful to guide attribution.
Reassuringly, there are several retrospective studies which have specifically evaluated rates of pneumonitis in ICI-treated patients who also received lung-directed radiation, finding no statistically significant difference in pneumonitis rates (43,45,49). In a secondary analysis of KEYNOTE-001, Shaverdian et al. found all grade pneumonitis rates of 8% vs. 1% (P=0.15) in patients who received thoracic-directed radiotherapy prior to ICI as compared to patients treated with ICIs alone (49). Hwang et al. retrospectively found all grade pneumonitis rates of 8% compared with 6% in patients previously treated with ICI combined with thoracic radiotherapy versus ICI alone (45). Interestingly, on multivariate analysis, they found that patients with an ir-AE had improved survival (HR 0.45, P=0.03) (45). Finally, the PACIFIC study, in which all patients received radiation to the lungs prescribed to at least 54 Gy administered 0–7 weeks before durvalumab, there was a modest numerical increase in any grade pneumonitis (34% vs. 25%) in patients that received durvalumab as compared to placebo that should, at least in part, be attributed to the rate of pneumonitis expected with durvalumab monotherapy (33). However, grade 3–4 pneumonitis rates were 3% in both arms (33).
Efficacy of combined treatment strategy
Local effects
Historically, chemotherapy and targeted agents have been used for radiosensitization to enhance the local effects of radiotherapy and to improve tumor control. While immunotherapies may not act as a radiosensitizer in a classical sense, preclinical and clinical evidence suggest that radiation is less effective when treating cancers in a state of immunosuppression (64). There are not yet any randomized trials in patients with metastatic disease comparing the local control rates of radiotherapy either administered alone or in combination with ICIs. In the setting of stage III NSCLC, the PACIFIC trial demonstrated an overall response rate of 28.4% compared to 16.0% when adding consolidative durvalumab and a disease control rate of 82.2% vs. 71.8% (33).
In the metastatic setting, several retrospective studies have compared local control rates in patients treated with combination SRS and ICI and SRS alone. Silk et al. found higher local response rates of 27% compared to 9% when SRS patients also received ipilimumab (35). However, disease control rates trended in the opposite direction (59% with ipilimumab, 68% without). In the studies performed by Kaidar-Person et al. and Patel et al., local control rates at 1-year were also numerically lower in patients treated with ipilimumab (52% vs. 86%, P=0.07 and 71% vs. 92%, P=0.40 in the two studies respectively) (46,47). However, local control following SRS is challenging to assess, especially in the setting of increased rates of radionecrosis/pseudoprogression that could potentially be observed following SRS and ICI. Pseudoprogression is not well accounted for using standard RECIST criteria, and thus alternative response criteria (irRC, iRECIST) are being utilized (21,22). More specifically for neuro-oncology, a response assessment has been developed by the RANO working group (65).
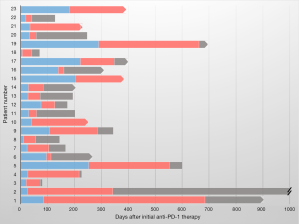
The local effects of radiotherapy could also be particularly valuable in metastatic patients who develop isolated sites of progression on ICI (66). The biologic explanation for this atypical pattern of resistance may be varied, but may, in some cases, be the result of localized loss of tumor antigens recognized by the immune system on ICI or other specific genetic changes mediating resistance (67-69). In cases such as these, focal radiation could ablate the area(s) of resistance, allowing for a sustained and more durable systemic response. Indeed, we found that among 59 patients who were treated with focal radiation for progression after starting a PD-1 inhibitor, 23 (39%) were able to continue to receive anti-PD-1 therapy for a median of 179 days (IQR 30–338, Figure 3) (48). In the future, better identification and biologic classification of patients with oligoprogression may further improve the utility of this combined treatment strategy.
Using radiotherapy to improve systemic immunotherapy effects
Given the favorable immunologic effects observed following radiation in vitro, one of the most provocative uses of radiotherapy being explored in the metastatic setting is to administer focal radiotherapy with the purpose of igniting a systemic immune response and overcoming either primary or acquired resistance to ICI. This work was encouraged by early case reports describing unusually favorable results obtained with the combination of radiation and ICI including a response outside of the radiation field (abscopal response) in a patient previously progressing on ipilimumab with biologic evidence of immune activation (18). Another case report demonstrated evidence of activity with ipilimumab combined with radiation in a heavily pretreated NSCLC patient (70)—a tumor type that does not generally respond to anti-CTLA-4 therapy. Although the PACIFIC study did not enroll metastatic NSCLC patients, this study also demonstrated an impressive PFS benefit with a hazard ratio of 0.52 (95% CI: 0.42–0.65, P<0.001) for the PD-L1 inhibitor durvalumab as compared to placebo in patients who received prior thoracic radiotherapy with concurrent chemotherapy (33). It remains unknown whether this more pronounced benefit for durvalumab, as compared to the benefit generally observed in the metastatic populations, was due to immune stimulating effects, eradication of gross disease prior to immunotherapy (10), or perhaps a combination of these two effects. Of note, patients who were randomized within 14 days of completing radiation demonstrated a more pronounced benefit to durvalumab as compared to placebo (HR for PFS 0.39, 95% CI: 0.26–0.58, as compared with HR 0.63, 95% CI: 0.49–0.80). A better understanding of the mechanisms underlying improved responses would help refine combined treatment strategies. Indeed, the existing radiation-immunotherapy studies mentioned above as well as other retrospective studies summarized in Table 2 are limited by significant heterogeneity regarding timing of radiation relative to ICI, sites and volume of disease to be radiated, and radiation dose/fractionation. Preclinical studies suggest these factors may impact results of combined radiation/ICI treatment significantly (71-74).
Conclusions
In summary, the combination of ICI and radiotherapy represents a promising treatment strategy for patients with metastatic cancers that is supported by preclinical evidence and early clinical data. Both prospective and an increasing amount of retrospective data suggest the combination of the two treatments is generally safe, although more study is needed to evaluate rare and late effects as well as to more accurately attribute toxicity to guide patient management. Additionally, potential increases in the rate of radionecrosis in patients receiving SRS and ICI must be balanced against the favorable clinical outcomes now observed with the combination of the two treatments (48), and is deserving of further study. In the meantime, the existing data is reassuring for the increasing number of patients who are candidates for ICI and palliative radiotherapy administered as standard of care treatment.
In addition, ongoing studies are evaluating the potential of a combined treatment strategy incorporating both radiotherapy and ICI to maximize benefit by addressing primary and acquired resistance to ICI, and by improving local control rates and palliative benefit achieved by radiation monotherapy. Existing clinical data suggests that radiotherapy can address localized progression in patients otherwise responding to immunotherapy; some of these patients can then continue to derive durable benefit from ICI. Unknown is whether the addition of radiotherapy to ICI will be able to reliably increase response rates as it has been demonstrated in preclinical models, and if so, the specifics of the patient, tumor and treatment parameters (type of ICI, timing, dose, fractionation and site of radiotherapy) required to maximize benefit.
Acknowledgments
None.
Footnote
Conflicts of Interest: JD Schoenfeld acknowledges research funding from BMS, Merck, and consulting fees from BMS, Debiopharm Group, AZ, Nanobiotix, and Tilos Therapeutics. Another author has no conflicts of interest to declare.
References
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal cell carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, van der Heijden MS, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Cohen EEW, Machiels EWC, Harrington KJ, et al. KEYNOTE-040: A phase III randomized trial of pembrolizumab (MK-3475) versus standard treatment in patients with recurrent or metastatic head and neck cancer. J Clin Oncol 2015;33:TPS6084. [Epub ahead of print]. [Crossref]
- Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311-9. [Crossref] [PubMed]
- Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med 2016;374:2542-52. [Crossref] [PubMed]
- Alexander BM, Schoenfeld JD, Trippa LT. Hazards of hazard ratios – deviations from model assumptions in immunotherapy. N Engl J Med 2018;378:1158-9. [Crossref] [PubMed]
- Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889-94. [Crossref] [PubMed]
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345-56. [Crossref] [PubMed]
- Motzer RJ, Tannier NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277-90. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomized, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. J Clin Oncol 2014;32:2913-19. [Crossref] [PubMed]
- Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018;18:313-22. [Crossref] [PubMed]
- Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced Type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014;41:843-52. [Crossref] [PubMed]
- Hiniker SM, Reddy SA, Maeker HT, et al. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys 2016;96:578-88. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]
- Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016;34:1510-7. [Crossref] [PubMed]
- Seymour L, Boaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Kwon ED, Drake CG, Scher HI, et al. Iplimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicenter, randomized, double-blind, phase 3 trial. Lancet Oncol 2014;15:700-12. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Tang C, Welsh JW, de Groot P, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res 2017;23:1388-96. [Crossref] [PubMed]
- Welsh JW, Tang C, de Groot P, et al. Phase II 5-arm trial of ipilimumab plus lung or liver stereotactic radiation for patients with advanced malignancies. Int J Radiat Oncol Biol Phys 2017;99:1315. [Crossref]
- Williams NL, Wuthrick EJ, Kim H, et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys 2017;99:22-30. [Crossref] [PubMed]
- Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018;36:1611-8. [Crossref] [PubMed]
- Theelen W, Peulen H, Lalezari F, et al. Randomized phase II study of pembrolizumab after stereotactic body radiotherapy (SBRT) versus pembrolizumab alone in patients with advanced non-small cell lung cancer: The PEMBRO-RT study. J Clin Oncol 2018;36:abstr 9023.
- Mcbride SM, Sherman EJ, Tsai CJ, et al. A phase II randomized trial of nivolumab with stereotactic body radiotherapy (SBRT) versus nivolumab alone in metastatic (M1) head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2018;36:abstr 6009.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Barker CA, Postow MA, Khan SA, et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res 2013;1:92-8. [Crossref] [PubMed]
- Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013;2:899-906. [Crossref] [PubMed]
- Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol 2016;27:434-41. [Crossref] [PubMed]
- Kiess AP, Wolchok JD, Barker CA. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys 2015;92:368-75. [Crossref] [PubMed]
- Colaco RJ, Martin P, Kluger HM, et al. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg 2016;125:17-23. [Crossref] [PubMed]
- Liniker E, Menzies AM, Kong BY, et al. Activity and safety of radiotherpayt with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology 2016;5:e1214788. [Crossref] [PubMed]
- Qin R, Olson A, Singh B, et al. Safety and efficacy of radiation therapy in advanced melanoma treated with ipilimumab. Int J Radiat Oncol Biol Phys 2016;96:72-7. [Crossref] [PubMed]
- Aboudaram A, Modesto A, Chaltiel L, et al. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: a safe and effective combination. Melanoma Res 2017;27:485-91. [Crossref] [PubMed]
- Anderson ES, Postow MA, Wolchok JD, et al. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer 2017;5:76. [Crossref] [PubMed]
- Bang A, Wilhite TJ, Pike LRG, et al. Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat Oncol Biol Phys 2017;98:344-51. [Crossref] [PubMed]
- Fang P, Jiang W, Allen P, et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neurooncol 2017;133:595-602. [Crossref] [PubMed]
- Hwang WL, Niemierko A, Hwang KL, et al. Clinical outcomes in patients with metastatic lung cancer treated with PD-1/PD-L1 inhibitors and thoracic radiotherapy. JAMA Oncol 2018;4:253-5. [Crossref] [PubMed]
- Kaidar-Person O, Zagar TM, Deal A, et al. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: the potential impact of immunotherapy. Anticancer Drugs 2017;28:669-75. [Crossref] [PubMed]
- Patel KR, Shoukat S, Oliver DE, et al. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol 2107:444-50. [PubMed]
- Pike LRG, Bang A, Ott P, et al. Radiation and PD-1 inhibition: favorable outcomes after brain-directed radiation. Radiother Oncol 2017;124:98-103. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Diao K, Bian SX, Routman DM, et al. Combination ipilimumab and radiosurgery for brain metastases: tumor, edema and adverse radiation effects. J Neurosurg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Gabani P, Robinson CG, Ansstas G, et al. Use of extracranial radiation therapy in metastatic melanoma patients receiving immunotherapy. Radiother Oncol 2018;127:310-7. [Crossref] [PubMed]
- Hubbeling HG, Schapira EF, Horick NK, et al. Safety of combined PD-1 pathway inhibition and intracranial radiation therapy in non-small cell lung cancer. J Thorac Oncol 2018;13:550-8. [Crossref] [PubMed]
- Martin AM, Cagney DN, Catalano PJ, et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Nardin C, Mateus C, Texier M, et al. Tolerance and outcomes of stereotactic radiosurgery combined with anti-programmed cell death-1 (pembrolizumab) for melanoma brain metastases. Melanoma Res 2018;28:111-9. [Crossref] [PubMed]
- Kroeze SG, Fritz C, Hoyer M, et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: a systematic review. Cancer Treat Rev 2017;53:25-37. [Crossref] [PubMed]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2017 Jul 21 -. Identifier NCT03223155. Concurrent or Sequential Immunotherapy and Radiation Therapy in Patients with Metastatic Lung Cancer (COSINR). 2018 Feb 21 [cited 2018 Jun 17]. Available online: https://clinicaltrials.gov/ct2/show/NCT03223155.
- Shibaki R, Akamatsu H, Fujimoto M, et al. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann Oncol 2017;28:1404-5. [Crossref] [PubMed]
- Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label phase 2 trial. Lancet Oncol 2012;13:459-65. [Crossref] [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Wu J, Hong D, Zhang X, et al. PD-1 inhibitors increase the incidence and risk of pneumonitis in cancer patients in a dose-independent manner: a meta-analysis. Sci Rep 2017;7:44173. [Crossref] [PubMed]
- Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res 2016;22:6051-60. [Crossref] [PubMed]
- Rodrigues G, Lock M, D’Souza D, et al. Prediction of radiation pneumonitis by dose-volume histogram parameter in lung cancer-a systematic review. Radiother Oncol 2004;71:127-38. [Crossref] [PubMed]
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119-42. [Crossref] [PubMed]
- Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114:589-95. [Crossref] [PubMed]
- Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 2015;16:e534-42. [Crossref] [PubMed]
- Gide TN, Wilmott JS, Scoyler RA, et al. Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Metastatic Melanoma. Clin Cancer Res 2018;24:1260-70. [Crossref] [PubMed]
- Anagnostou V, Smith KN, Forde PM, et al. Evolution of neoantigens landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov 2017;7:264-76. [Crossref] [PubMed]
- George S, Miao D, Demetri GD, et al. Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity 2017;46:197-204. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365-72. [Crossref] [PubMed]
- Young KH, Baird JR, Savage T, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One 2016;11:e0157164. [Crossref] [PubMed]
- Beyranvand Nejad E, Welters MJP, Arens R, et al. The importance of correctly timing cancer immunotherapy. Expert Opin Biol Ther 2017;17:87-103. [Crossref] [PubMed]
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379-88. [Crossref] [PubMed]
- Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618. [Crossref] [PubMed]


