Defining the radiation oncologist’s role in palliative care and radiotherapy
Independent of disease site, location of practice, and type of practice, nearly all radiation oncologists take care of advanced cancer patients. Approximately 40% of advanced cancer patients receive palliative radiotherapy (1), and 40% of radiotherapy is delivered with palliative intent (2). Most radiation oncology clinics do not have formalized or dedicated palliative radiotherapy programs, which may have the infrastructure and resources to deliver timely multi-disciplinary palliative and supportive care. However, given the ubiquity and complexity of advanced cancer patients with symptomatic disease, all radiation oncologists should be comfortable providing general palliative care for these patients—a belief that was vocalized by respondents in a recent survey of American Society for Radiation Oncology (ASTRO) members (3).
The radiation oncologist’s role in the palliative care team
Patients with advanced cancer have complex and multi-faceted needs, including those related to physical symptoms and to emotional, social or spiritual distress. With progressive disease, a patient’s needs may evolve; questions surrounding prognosis and end of life (EOL) care may surface. Given the different skill sets required to provide effective, holistic care for advanced cancer patients, a team-based approach is employed for pain/symptom management, social/spiritual issues, prognosis discussion, and goals of care. A palliative care team is a “comprehensive group of specialized clinicians from a variety of disciplines who share a common goal to improve the quality of life for patients and families facing serious illness” (4). Palliative care teams providing care to patients and families, though variable by institution, generally are comprised of palliative care physicians, who receive specialized training in advanced communication skills and treatment of complex symptom management; nurses; social workers; pharmacists; chaplains; and the patient’s medical, radiation, and surgical oncologists. Palliative care teams may be distinguished as generalists, which include oncology clinicians and nurses that provide primary palliative care, versus specialists, which include clinicians that have received specialized training in provision of palliative care. Among a survey of ASTRO members, 79% respondents reported having a palliative medicine service at their institution (3). This number will likely increase over time, as palliative care becomes integrated as part of the global service of every hospital or clinic treating cancer patients (5).
By virtue of a radiation oncologist’s training and day-to-day multi-disciplinary approach to treating curative-intent, cancer patients, radiation oncologists already have the know-how and are familiar with the benefits of multi-disciplinary interaction. Radiation oncologists provide effective palliation of multiple symptoms from advanced cancer (see clinical indications for palliative radiotherapy). As patients may have several radiation oncology visits over a short time, radiation oncologists also have the unique opportunity to provide basic supportive care (e.g., nausea, pain, constipation) and screen patients, who may require more complex symptom management and/or psychosocial support. Many patients considered for palliative radiotherapy have concomitant symptoms. For example, approximately 10–15% of palliative radiotherapy patients have concurrent depression/anxiety (6,7). This strategy of screening and referring to appropriate team members has been associated with improved patient symptoms including fatigue, depression, anxiety, drowsiness, and well-being among patients seen in a palliative radiotherapy clinic (8). In this model, the patient’s primary oncology providers—radiation oncologists, medical oncologists, and oncology nurses included—provide general palliative care and refer patients to a palliative care specialist if/when more complex needs arise.
Radiation oncologists can also educate members of the palliative care team regarding indications for and mis-conceptions of palliative radiotherapy. This important interaction may improve patient care, in particular with referral of patients that palliative medicine specialists or oncologists did not otherwise consider radiotherapy for and/or referral of patients at an earlier time point.
Estimating life expectancy for patients and treatment recommendations
For advanced cancer patients, referral for palliative radiotherapy may herald progressive disease. In these moments, patients may reflect on the trajectory of their cancer treatment and inquire about prognosis. As providers who see patients at these critical moments, radiation oncologists must be comfortable with estimating life expectancy to effectively counsel patients and to determine a patient’s likelihood of benefiting from palliative radiotherapy. Furthermore, in patients that are deemed to be palliative radiotherapy candidates, radiation oncologists must choose a dose/fractionation scheme that balances the patient’s life expectancy with long-term efficacy and side effects (9,10). In fact, among an international survey of radiation oncologists, patient life expectancy was the factor most frequently influencing dose/fractionation prescription (11).
Despite this, physicians are generally poor at prognosticating (12) and tend to be optimistic in survival estimates, especially for patients with limited life expectancy (13,14). This is significant as overestimates of patient life expectancy may contribute to patients receiving a proportionally long radiation treatment course to their remaining life span (15,16).
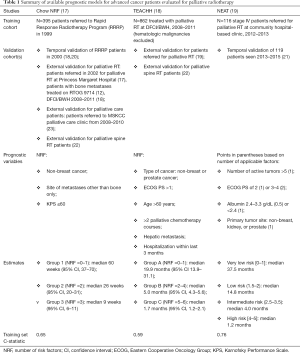
Realizing that prognostication is complex, several groups over the last decade have developed prognostic models for palliative radiotherapy patients (Table 1). These models provide quantitative estimates of how various prognostic factors impact patient life expectancy. Prognostic models developed for advanced cancer patients receiving palliative care (24) are as accurate or more accurate than physician estimates. The available prognostic models for advanced cancer patients being evaluated for palliative radiotherapy vary in regard to the time frame of the training cohort [e.g., 1999 for Chow number of risk factors (NRF) model (17); 2008–2011 for TEACHH model (18); and 2012–2013 for NEAT model (19) and the setting in which patients were evaluated (e.g., in academic versus community clinics)]. While subtle, they are important to consider when evaluating whether a prognostic model may be generalizable to a patient of interest.
Full table
Regardless, for each model a training cohort was evaluated to identify a set of prognostic variables that were significantly associated with patient survival. Most models use a point system in which each significant adverse prognostic factor is assigned a value or counted. The sum of points or number of risk factors determines which group a patient falls into, with each group having an estimated survival. While it is beyond the scope of this review to discuss development of a prognostic model, it is important to note that each model was validated to confirm that the model can predict for survival in other cohorts separated by time (temporal validation) or location (external validation). The three summarized models—Chow NRF, TEACHH, and NEAT models—have relatively high C-statistics varying from 0.59 to 0.76, reflecting goodness of the model; a C-statistic of 0.5 suggests that the model is no better than a flip of a coin with predicting an outcome, while 1 suggests that the model perfectly predicts those who will have a certain outcome and those who will not.
The Chow NRF model has been the most extensively validated and is the simplest, having just 3 factors to consider. Notably, in all three models, performance status is a significant predictor for survival. Indeed, performance status has been found to be most strongly correlated with survival (12) and highlights the importance of clinical evaluation of these patients. Newer therapies such as immunotherapy are emerging, which may alter cancer progression and mortality. While all three prognostic models were developed prior to wide spread use of immunotherapy, it is helpful to note that the Chow NRF model, which was developed among patients seen in 1999, still performs quite well among more contemporary cohorts (19,21,22), who inevitably are being exposed to newer systemic agents.
Prognostic disclosure and communication
Recent guidelines from American Society of Clinical Oncology (ASCO) advocate that “clinicians should reassess a patient’s goals, priorities, and desire for information whenever a significant change in patient’s care is being considered” (25). Triggers for prognostic disclosure and goals of care discussions will occur throughout a patient’s illness, including at diagnosis, relapse or progression, change in treatment approach, and/or at the patient’s and family’s request. At each of these junctures, the physician should ascertain the patient’s interest in disclosure and the how much information is desired, if so.
Many patients are referred for consideration of palliative radiotherapy in the setting of relapsed or progressive disease and at times when treatment approaches (e.g., systemic therapy) are being changed. Radiation oncologists therefore shoulder the responsibility for prognostic disclosure and assessing a patient’s goals to appropriately tailor radiation treatment recommendations, but also to address questions from advanced cancer patients, who may be encountering uncertainties with their disease course.
Goals of communication
Gaps in physician-patient communication may contribute to patients’ misunderstandings of the goals and limitations of their care. Among 1,193 newly diagnosed patients with stage IV lung or colorectal cancer, 69% of lung cancer and 81% of colorectal cancer patients had inaccurate understandings of whether palliative chemotherapy would cure their cancer (26). Similar findings were seen in a subset of this cohort, who were surveyed about their expectations on palliative radiotherapy. Among 384 irradiated patients with wet stage IIIB or IV lung cancer, 78% believed that radiotherapy was very or somewhat likely to help them live longer and 43% felt that radiotherapy was very or somewhat likely to cure their cancer. Not surprisingly, 92% of patients with inaccurate beliefs on the curative potential of radiotherapy also had inaccurate beliefs about chemotherapy (27). The frequency of prognostic disclosure by the treating physician was not captured in these studies, but interestingly, one of the independent predictors of patients with inaccurate beliefs of chemotherapy was a patient’s very favorable rating of their physician’s communication (26). These findings suggest that there is room for physicians to improve their patients’ understanding of palliative treatment, but this may potentially come at the cost of patient satisfaction.
Physician prognostic disclosure and communication also provides patients a realistic framework of their life expectancy, upon which patients can provide informed consent or dissent for treatment, frame their goals of care, and enhance communication with their family. Like physicians, patients may base treatment preferences on perceived life expectancy. Recall of prognostic disclosure has been associated with more accurate patient-derived life expectancy estimates. Longer (less accurate) patient-derived life-expectancy estimates are associated with preference for life-extending care and lower likelihood of a do-not-resuscitate order (28). These findings suggest that more accurate awareness of life expectancy permits patients to tailor their EOL care accordingly. Indeed, patients who recognize that their cancer is terminal are more likely to prefer symptom-directed care over life-extending care and to ultimately receive EOL care that is consistent with their baseline preferences (29). Notably, a small subset (~20%) of patients that understand their imminent mortality from cancer may still choose to receive life-extending care (30). Prognostic disclosure and accurate understanding of prognosis is also important for patients and caregivers to better support each other through enhanced patient-family communication (30) and to prepare caregivers emotionally and logistically for the possibility of the patient’s death (31).
Misconceptions of prognostic disclosure and/or EOL discussions
Physicians are often hesitant to disclose prognosis, given concerns of potentially damaging a patient’s hope or provoking emotional distress, fear of being blamed, fear of confronting their own emotions, and/or instilling a sense of abandonment. Physicians’ fears are not completely unfounded: patients perceive physicians that deliver more optimistic messages as having more compassion (32). However, multiple studies with patients spanning different countries (30,33), stages of disease (early versus advanced) (34), and age (35) have documented that most patients desire prognostic disclosure (28). Among over 2,000 patients across 34 UK hospitals, 87% of patients preferred to have as much information as possible, both good and bad (33). A similar proportion of adolescent and young adults (AYA) with cancer (83%) considered prognostic information to be extremely or very important, although patients with a lower likelihood of cure (<50%) were less likely to consider prognostic information important (35).
While oncologists are adequately aware of their patient’s desire for information on side effects from treatment and likelihood of tumor response, identifying the amount of prognostic information to disclose is more challenging (36). Although cancer patients desire prognostic disclosure, patients may prefer that the oncologist first confirm whether they would like this information (34). Indeed, among AYA cancer patients, 10% considered knowing about the likelihood of cure to be extremely or very upsetting (35). Based on this, it may be helpful to first give a patient a preview of the type of information available, ask whether the information is desired, and then follow the patient’s request.
Contrary to commonly-held beliefs, disclosing prognosis to cancer patients is not associated with increased anxiety (28,35,37,38), depression (39), worry (39), or decreased hope (28,38,40) among cancer patients. In fact, in a study evaluating surrogate decision makers’ attitudes toward balancing hope and honesty with prognostic disclosure, 93% felt that avoiding discussions about prognosis was an unacceptable way to maintain hope (31). Moreover, prognostic disclosure does not appear to be associated with decreased patient-derived ratings of patient-physician relationship (28).
Potential benefits of communication
As previously alluded to, clear, honest communication may provide the patient critical information to inform his treatment decisions, goals of care, and planning. Within pediatric oncology, data also suggests that in addition to content, the process of communication may in of itself engender hope, peace of mind, and trust. Among 353 parents of children with newly diagnosed cancer, high-quality physician communication, as rated by the parent, was associated with greater rated trust of the oncologist, peace of mind, and decreased anxiety (38). Even in the setting of poor prognoses, receipt of high-quality information was associated with greater peace of mind and communication-related hope among parents of children in which a chance of cure was <75% (38). It is notable that among this cohort, accurate understanding of prognosis was not significantly associated with parental report of high-quality information or high-quality communication from the oncologist. Therefore, while any bad news can be difficult, patients and family members still find hope in the process of communication. Last, facilitating prognostic disclosures and EOL discussions have been associated with earlier hospice referrals and less aggressive medical care near death. The latter is notable as aggressive medical care at the EOL is associated with worse patient quality of life and worse bereavement adjustment among caregivers (39).
Patient preferences of communication
Like other scenarios of delivering bad news (41), most patients prefer physicians to obtain permission prior to disclosing information (34). Most patients prefer physicians to be realistic, provide opportunities to ask questions, and approach the patient as an individual when discussing prognosis (34,42). In a study of inpatients’ preferences on EOL communication with physicians, two major themes surfaced. Patients want their physician to “know me” and acknowledge the influence of family roles and life history on a patient’s values and priorities. In addition, patients desire “conditional candor” from physicians, a process of assessing the patient’s readiness, being invited to the conversation, and delivering information with sensitivity (43).
Instilling hope
Hope is a broad concept, which can hold different meanings for each individual. In a written survey of 126 patients with metastatic cancer, patients were asked their definition of hope through provision of four exemplars and the option of free text. The most commonly endorsed exemplar (although only by 19% of the cohort) was “that you can still enjoy a good quality of life even if life expectancy is uncertain.” The majority of patients (62%) wrote their own definition with or without also picking a given exemplar (34). In this same survey, patients were queried on physician behaviors that were hope-giving. Most frequently rated behaviors included physicians offering the most up to date treatment, appearing to know all there is to know about the patient’s cancer, reassuring the patient that the pain will be controlled, and reviewing all treatment options (34). Similar themes have also emerged in interview-based studies of advanced cancer patients and their caregivers. Patients felt that by emphasizing what can be done (e.g., controlling physical symptoms, emotional support), exploring realistic goals, and discussing day-to-day living, physicians can still foster coping and nurture hope even when discussing prognosis and EOL issues (44). Strategies to foster hope when discussing prognosis and EOL issues are summarized in Table 2. In this issue, Dharmarajan et al. also reviews communication skills in palliative radiation oncology.

Full table
Clinical indications for palliative radiotherapy
Uncontrolled, progressive tumor growth in advanced cancer patients may be associated with pain, neurologic impairment, bleeding, ulcerative mass, obstruction of luminal organs, or other functional impairments. The efficacy and safety of radiation therapy for palliating these symptoms is well established in multiple settings, including those with loco-regionally advanced, inoperable, metastatic, or previously irradiated recurrent tumors (45). Optimizing the therapeutic ratio of palliative RT in this heterogeneous patient population requires detailed assessment of symptomatology, tumor characteristics, and accurate prognostication by the radiation oncologist.
Painful bone metastases
Bone is the third most common site of cancer metastases after lung and liver (46). Bone metastases may be associated with various local complications, varying from pain at the site of metastases, pathological bone fracture, to compression of the spinal cord or nerve roots (47). Based on a meta-analysis of 25 randomized trials, palliative RT is highly efficacious for pain control, with 60–80% achieving improvement of pain within 3–4 weeks (48).
Single-fraction radiation therapy provides excellent pain control for uncomplicated bone metastases (48). In general, uncomplicated bone metastases refer to lesions without an associated large soft tissue mass, have low risk of imminent fracture (i.e., no planned surgical fixation), no evidence of spinal cord or cauda equina compression, and not previously irradiated (49). Rates of efficacy and durability of pain control with single-fraction radiation are equivalent to more protracted radiotherapy courses (i.e., ≥5 fractions) (50-52). While rates of retreatment may be higher with single- versus multi-fraction RT (52,53), it is unclear whether this is secondary to physician comfort with retreating after a lower initial RT dose versus lower rates of durability. In addition to cost and resource utilization, single fraction treatment allows patients to undergo the planning procedure and RT delivery on the same day. For many patients, especially those with poor performance status, this maximizes both convenience and delivery of an effective treatment (54-56).
Palliative RT for bone metastases is well tolerated (48). Specific side effects depend on the irradiated anatomical site, size of radiation field, and radiation dose. Transient fatigue, pain flare, mild nausea and mild local skin erythema are commonly reported. Inclusion of the upper gastrointestinal tract may be associated with transient odynophagia and nausea, while diarrhea may be noted with bowel irradiation. These are usually self-limiting (57,58). Frequency and severity of side effects are lower with single fraction versus multi-fraction palliative RT (59).
However, in certain scenarios multi-fraction RT may be preferred to single fraction RT, including bone metastases causing neuropathic pain (51) or associated with an extra-osseous soft tissue mass (60). Studies suggest longer durability of pain control with a multi-fraction regimen. Patients irradiated after surgical fixation of bone metastases have not routinely been included in prior randomized trials comparing single- to multi-fraction RT; a longer RT course has historically been performed (47).
Palliative RT can be given in repeated courses to different sites of the body. Re-irradiation of a bone metastasis for recurrent pain after prior palliative RT may also be feasible, depending on the location, prior palliative RT dose, and time between RT treatments (61). Randomized data by Chow et al. suggest that a single-fraction RT for retreatment of painful bone metastasis results in similar efficacy and safety compared with multi-fractions RT (55). Retreatment can be given 1 month after the initial treatment if the response was not optimal (55).
Spinal metastases
Spinal vertebrae are the most frequently affected sites for bone metastases. In addition to minimizing pain, adequate control of spine metastases may help preserve the mechanical integrity of spinal column and prevent malignant spinal cord compression (MSCC) (62). While skeletal related events of asymptomatic spinal metastases may be reduced by bone modifying agents [e.g., bisphosphonate or denosumab (63) or radio-nucleotide treatment (64), symptomatic spinal metastases should be assessed by radiation oncologists for consideration of radiation therapy].
External beam palliative RT is the standard therapy for symptomatic spinal metastasis (62). Surgery is also an important option for selected patients with expected survival over 3 months (65). In patients with limited survival of 6 months or less, hypofractionated multi-fraction palliative RT prevented severe complications including vertebral compressive fractures, cord compression or neurological deterioration in more than 90% of irradiated patients (66). Multi-fraction RT is also associated with higher remineralization rates of irradiated vertebrae, compared with single-fraction RT (67). Improved mechanical strength of the spinal column could potentially prevent future complications.
Mechanically unstable spinal metastases are associated with higher risk of failure after RT (68) and compared to “uncomplicated” spinal metastases, nearly a 3-fold increased risk of adverse spinal events such as cord compression, pathologic fracture, or need for salvage surgery (66). Classification of spine instability is facilitated by the “Spinal Instability Neoplastic Score” (SINS; Table 3) (70). The SINS is a composite score (range 0–18) of 6 sub-scores: spine location, nature of bone pain, morphology of the bone lesion, spinal alignment, extent of vertebral body fracture, and involvement of posterolateral spinal elements. SINS has good content validity and excellent inter-observer and intra-observer reliability among surgeons and radiation oncologists (71), hence can be used as a common language among surgeons and radiation oncologists. SINS should be routinely assessed for each patient referred for consideration of spinal RT. This can help identify patients who will likely respond poorly to RT alone and may benefit from referral to a spine surgeon for consideration of spine stabilization (72) or an interventional procedure like kyphoplasty (73).
Retreatment of a progressing, previously irradiated spinal metastasis can be challenging given that the spinal cord is radiosensitive (74,75) and at risk of radiation myelopathy and irreversible paralysis. Cohort studies, as well as a randomized controlled study, have shown that reirradiation with conventional RT techniques is safe if the cumulative biological equivalent dose (BED with alpha/beta =2) is in the range of 100–135 Gy, time between radiotherapy treatments is >6 months, and the BED dose of each course is ≤98 Gy (55,61,76). However, to respect the cumulative tolerance of the spinal cord, the RT dose used for re-irradiation is often lower than what was used in the first RT course, which may lead to unsatisfactory clinical outcome and disease control after reirradiation (76).
Stereotactic body radiation therapy (SBRT), a highly conformal radiation technique that employs high dose per fraction with near-rigid patient immobilization, has been increasingly used for spine metastases (77). Because of the 1–1.5 mm accuracy achieved through patient immobilization, image-guided RT, and steep dose fall off, SBRT is an attractive alternative to conventional RT (78). SBRT can achieve high, ablative radiation doses for spinal tumor control and at the same time, geographically spare the spinal cord from the damage of re-irradiation (62). While radiation myelopathy has been reported after SBRT re-irradiation (79), findings from Sahgal and colleagues suggest that >5 months interval between conventional palliative RT and SBRT re-irradiation, limiting the maximum point dose to the thecal sac to nBED 20–25 Gy (2/2) appears to be safe as long as the cumulative point max to the thecal sac is ≤70 Gy (2/2) and that the SBRT thecal sac point max dose does not comprise more than 50% of the total cumulative dose (79).
Early experience with spinal SBRT has demonstrated impressive local control results and favorable side effects profile (80). One-year local control rates of 90% or higher are achievable in multiple series across different histologies (81). As such, there is interest to use spine SBRT for initial RT treatment. An ongoing multi-center randomized controlled trial (82) is comparing spinal SBRT against single fraction palliative spinal RT in upfront setting. Recent data from a phase II randomized trial comparing SBRT (24 Gy/1 fraction) with conventional palliative RT (30 Gy/10 fractions) demonstrated quicker and improved pain response with SBRT (83). These and ongoing work are important to define the clinical efficacy, toxicity, and cost-effectiveness of spine SBRT. We caution against unselected use of SBRT for all spinal metastases, especially amongst patient with limited life expectancy, since conventional palliative RT is supported by high-level evidence, high efficacy, and lower cost (84).
Cord compression
Assessment of a patient with MSCC can be summarized with the mnemonics “NOMS” (85). Neurological (N) examination is essential to correlate findings with the spinal magnetic resonance imaging (MRI). Degree of cord compression can be classified by the Bilsky score (86). Bilsky grade II to III cord compression on MRI warrants consideration of urgent surgical decompression (87). The baseline lower limb function assessment by Frankel score (88) can aid subsequent review of treatment outcome.
Oncological “O” assessment of tumor radio-sensitivity may help identify those patients (e.g., those with radio-resistant tumors) that may benefit from a more aggressive surgical approach. Mechanical “M” stability should be as assessed by the SINS (Table 3). Last, systemic “S” assessment of a patient’s co-morbidities and life expectancy may help identify who may benefit from more aggressive local treatment. Discussion of treatment options should ideally occur in a multi-disciplinary setting (89) with a spine surgeon, primary oncologist, radiation oncologist and palliative care physician.
For patients with radioresistant tumors, neurologic compromise, unstable spinal mechanics, and a reasonably long life-expectancy, an aggressive approach with surgical decompression, stabilization, and radiation therapy should be considered (65). The landmark study by Patchell et al. (90) demonstrated superior lower limb function, preservation of continence and overall survival when surgery was performed prior to radiation therapy (RT).
In contrast, for patients presenting with >48 hours of with paralysis, limited life expectancy (≤3 months), multiple levels of spine involvement, conservative treatment with RT alone is appropriate (91). In a randomized comparison of single- versus multi-fraction RT for MSCC in patients with limited life expectancies (SOCRAD III), rates of overall survival were similar, in addition to ambulatory status at 8 weeks (92).
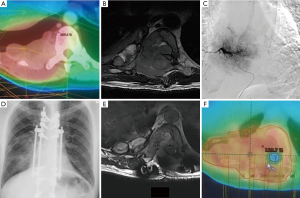
In the acute cord compression setting, SBRT for radiosurgical decompression (62) is still considered investigational, since a physical distance of 2–5 mm between the tumor and cord is necessary to achieve adequate epidural tumor coverage (93). Separation surgery, which clears the epidural component of the tumor, can help achieve this distance (94). SBRT can then safely ablate the residual tumor in vertebral body and paraspinal areas. This type of post-operative spinal SBRT is technically challenging due to metallic implant artifacts that may obscure organ localization and compromise dosimetry (93). Close collaboration between spinal surgeons, radiologists, radiation oncologists and medical physicists is necessary. Figure 1 was an illustrative patient who benefited from this multidisciplinary approach.
Brain metastases
Brain metastases affect around 40% of advanced cancer patients (95). This is a very heterogeneous group of patients with widely different survival estimates (96-98). On one end of the spectrum, these include patients with poor performance status, uncontrolled extracranial disease, high volume brain metastases not amenable for surgical or radiosurgical treatment (OS ≤3 months). The other end of the spectrum may instead include breast cancer patients with a single, small volume brain metastasis and minimal extracranial disease burden (OS >2 years). Treatment should therefore be personalized based on patient performance status, disease histology, and prognostication.
Whole brain radiation therapy (WBRT) is the conventional therapy for brain metastases (99). It provides transient control for brain metastases and helps to decrease the development of new metastases after focal therapies. However, no clinical trial has demonstrated a survival benefit with WBRT. Associated side effects including alopecia, scalp dermatitis, and neurocognitive function impairment may lead to significant deterioration in quality of life, especially in the first 3 months post-WBRT (100,101). Recently, the QUARTZ trial (102), a non-inferiority trial that randomized patients with brain metastases from lung cancer to WBRT versus supportive care, demonstrated that WBRT was associated with a benefit, but of only a mean quality-adjusted life years (QALY) of 4.7 days. While supportive care was not shown to be non-inferior to that of WBRT, many believe that a mean QALY of 4.7 days is clinically not meaningful.
Focal therapies, including radiosurgery and surgery, are associated with higher local tumor control rates compared with WBRT (103) and are considered for patients with expected survival longer than 3 months (104). Brain metastases larger than 3 cm may benefit from surgery over radiosurgery given higher rates of local control and more rapid relief of edema (103). Focal treatment alone (i.e., without WBRT) requires routine surveillance MRI scans to monitor for new distant brain metastases that may require salvage treatment. Risk of new distant intracranial disease is approximately 50% or higher at 6 months (105,106).
Recent advances in RT techniques have increased the therapeutic ratio of cranial RT. Multiple randomized controlled trials confirm the role of post-operative surgical cavity radiosurgery, which spares patients from the neuro-toxicity of WBRT (106-108). Hypofractionated stereotactic radiotherapy (SRT) for large volume brain metastases is also efficacious based on prospective cohort studies (109,110). Given that relatively low doses to the hippocampus are associated with neuro-cognitive decline (111), hippocampal sparing WBRT (112) has been studied using advanced RT planning techniques such as intensity modulated radiotherapy. Preliminary results on neurocognitive preservation showed superior outcome compares with a historical cohort (113).
Beside advanced RT techniques, the use of memantine, an oral NMDA receptor antagonist, had been shown to moderately delay time to cognitive decline and reduce the rate of decline in memory, executive function, and processing speed in patients receiving WBRT (114). It is a well-tolerated drug with minimal toxicity but a prolonged course of treatment of 24-week is needed.
Thoracic malignancies
RT has been used to relieve symptoms caused by tumor’s compressive effect on thoracic structures (115), including major airway invasion (trachea and/or bronchus) causing dyspnea, cough, and hemoptysis. Tumors compressing the superior vena cava may cause neck, face and upper limb edema and shortness of breath. Large tumor or bulky mediastinal lymph nodes may cause esophageal obstruction. Tumor direct invasion in brachial plexopathy by apical tumor may cause severe pain and limb weakness.
Fourteen randomized clinical trials evaluating palliative RT for lung cancer have been performed and were summarized in meta-analysis (116). Various dose fractionation regimens had been tested, including short (10 Gy in 1 fraction, 17 Gy in 2 weekly fractions or 20 Gy in 5 daily fractions) and long RT courses (30–45 Gy in 10–15 daily fractions). In general, a high proportion of patients achieve satisfactory symptomatic relief, especially for cough and hemoptysis. Short course RT are highly effective for symptom relief with relatively mild side effects (117). This is an attractive option for patients with poor performance status and limited survival expectancy. Those with better performance status may benefit from longer course RT (118) given association of potentially improved overall survival, albeit at the cost of higher toxicity, mainly esophagitis (46).
Gastrointestinal malignancies
Obstructive and bleeding symptoms of locally advanced gastrointestinal tract cancer can be very distressing to patients and caregivers. As these patients usually have limited overall survival, a brief RT course is preferred. Single fraction treatment, 1-week treatment (20 Gy in 5Fr) to 2-week treatment (30 Gy in 10Fr) have all been commonly employed in clinical practice (119).
For cancer of esophagus, dysphagia may be addressed through stenting of the obstructed site (120), although the tumor may grow through or around the stent lumen in more than one-third of patients. Hypofractionated palliative RT (27 Gy in 6 fractions in 3 weeks) to the esophagus can be used to relieve dysphagia in nearly 80% of patients (121). Median duration of relief was 24 weeks (122).
Bleeding and gastric outlet obstruction from gastric cancer may be palliated with a brief course of RT (1 to 5 fractions) in patients with poor performance status. Time to relief is rapid (123). Delivery of higher dose using 3D conformal or intensity-modulated radiation therapy (IMRT) techniques may be considered in patients with satisfactory performance status and is associated with improvement in bleeding, obstructive symptoms, and/or pain in more than 70% of cases (124).
Locally advanced or recurrent rectal cancer may present with bleeding, pain on defecation, sacral plexus neuropathic pain, mucous discharge, infections or fistula formation. Palliative RT to pelvis, either with conventional fractionation (45 Gy in 25 fractions) or hypofractionated RT (30 Gy in 6 fractions) both can give satisfactory symptom pain relief in ~70–90% of patients, decrease bleeding in more than 70% of patients, and control tumor growth in about 50% (125,126). However, the duration of the disease control after external beam RT is short (~3–9 months). Dose escalation with high dose rate intra-luminal brachytherapy may be a promising way to improve duration of tumor control (127).
Primary palliative care assessment by radiation oncologists
As pain control is a frequent indication for palliative RT referral, radiation oncologists should be familiar with diagnosing and managing pain. Patient-reported questionnaires have been proposed for daily clinical use. The Edmonton Symptom Assessment System (ESAS) (128) is of the most popular and well validated scoring systems. It assesses 9 symptoms—pain, fatigue, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath—on a scale of 0 to 10. Changes in physical, emotional, and total symptom distress scores have been validated with clinically significant symptom improvement or deterioration (129). ESAS can be conveniently integrated routinely into radiation oncology clinic session (130). Persistent high scores warrant early review and referral to palliative care specialist for further evaluation.
As pain relief should be achievable in 60–80% of patients after RT (48), assessment of a patient’s opioid regimen should be performed before, during and after palliative RT. Universal screening for patients on opioid is recommended. Patients who are younger; male; and had a mental health or substance abuse disorder, a history of alcohol abuse, or a history of tobacco use are at greater risk for aberrant opioid use (131). Two simple screening tools for substance dependence include the CAGE-AID questionnaire (132), which has been adapted to include drug use, and the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) (133).
Patients that test positive on CAGE-AID or SOAPP-R should have their pain levels regularly monitored. Aberrant behavior, such as complaints of pain inconsistent with disease status or repeated reports of lost opioid medications, should be red flags. Referral of these patients to a specialist palliative care physician may be warranted to address pain safely.
Depression may also be readily screened in the radiation oncology clinic. Up to 10–15% of patients undergoing RT report depression and/or anxiety (6,7). The Patient Health Questionnaire (PHQ-9) (134) is a validated instrument for screening patients who may benefit from referral to the psycho-oncology service. PHQ-9 is a 9-item scale with a possible score of 0–27. Scores >9 should prompt screening for symptoms of depression and consideration of a psycho-oncology specialist referral. Patient should be re-evaluated for progress during follow-up at the radiation oncology clinic with the aim of symptom remission within 3 months.
Last, spirituality assessment is recognized as one of the domains of high-quality palliative care (135). Spirituality is an integral dimension of human beings and has been recognized as a critical factor in the well-being of patients. Unresolved spiritual distress can lead to poor quality of life and poor health outcome (136). While detailed assessment and provision of spiritual care should be referred to chaplains, basic spiritual distress screening can be done with relatively simple assessment tools such as “FIGA” (137,138), a validated tool, or “Hope” (139) and “Spirit” (140). Radiation oncologist should be able to address spiritual issues of the patient, diagnose and facilitate early treatment of spiritual distress and integrate patients’ spiritual resources of strength into the treatment plan.
Conclusions
Radiation oncologists play an important role in the holistic care of advanced cancer patients. Through collaboration with multidisciplinary care team, radiation oncologist can contribute substantially in the overall care plan, including communication, prognostication, and provision of radiotherapy to palliate local effect of tumor progression. Radiation oncologists are also primary palliative care providers, monitoring and managing symptoms, and screening for distress (Table 4). By attending to all dimensions of a patient’s suffering, radiation oncologists can provide compassionate care to improve the quality of life of patients and their caregivers.

Full table
Acknowledgments
The authors thank the multidisciplinary palliative care team co-working with them throughout the years, as well as the palliative care mentors who have nurtured their career development.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Murphy JD, Nelson LM, Chang DT, et al. Patterns of Care in Palliative Radiotherapy: A Population-Based Study. J Oncol Pract 2013;9:e220-7. [Crossref] [PubMed]
- Janjan NA. An Emerging Respect for Palliative Care in Radiation Oncology. J Palliat Med 1998;1:83-8. [Crossref] [PubMed]
- Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: Report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol 2017;7:113-9. [Crossref] [PubMed]
- Tuggey EM, Lewin WH. A multidisciplinary approach in providing transitional care for patients with advanced cancer. Ann Palliat Med 2014;3:139-43. [PubMed]
- Jordan K, Aapro M, Kaasa S, et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol 2018;29:36-43. [Crossref] [PubMed]
- Frick E, Tyroller M, Panzer M. Anxiety, depression and quality of life of cancer patients undergoing radiation therapy: a cross-sectional study in a community hospital outpatient centre. Eur J Cancer Care (Engl) 2007;16:130-6. [Crossref] [PubMed]
- Hahn CA, Dunn R, Halperin EC. Routine Screening for Depression in Radiation Oncology Patients. Am J Clin Oncol 2004;27:497-9. [Crossref] [PubMed]
- Pituskin E, Fairchild A, Dutka J, et al. Multidisciplinary team contributions within a dedicated outpatient palliative radiotherapy clinic: a prospective descriptive study. Int J Radiat Oncol Biol Phys 2010;78:527-32. [Crossref] [PubMed]
- Rades D, Lange M, Veninga T, et al. Final Results of a Prospective Study Comparing the Local Control of Short-Course and Long-Course Radiotherapy for Metastatic Spinal Cord Compression. Int J Radiat Oncol Biol Phys 2011;79:524-30. [Crossref] [PubMed]
- Rades D, Panzner A, Dziggel L, et al. Dose-escalation of whole-brain radiotherapy for brain metastasis in patients with a favorable survival prognosis. Cancer 2012;118:3852-9. [Crossref] [PubMed]
- Fairchild A, Barnes E, Ghosh S, et al. International Patterns of Practice in Palliative Radiotherapy for Painful Bone Metastases: Evidence-Based Practice? Int J Radiat Oncol Biol Phys 2009;75:1501-10. [Crossref] [PubMed]
- Chow E, James JL, Hartsell W, et al. Validation of a Predictive Model for Survival in Patients With Advanced Cancer: Secondary Analysis of RTOG 9714. World J Oncol 2011;2:181-90. [PubMed]
- Chow E, Davis L, Panzarella T, et al. Accuracy of survival prediction by palliative radiation oncologists. Int J Radiat Oncol Biol Phys 2005;61:870-3. [Crossref] [PubMed]
- Gripp S, Moeller S, Bolke E, et al. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol 2007;25:3313-20. [Crossref] [PubMed]
- Gripp S, Mjartan S, Boelke E, et al. Palliative radiotherapy tailored to life expectancy in end‐stage cancer patients. Cancer 2010;116:3251-6. [Crossref] [PubMed]
- Guadagnolo BA, Liao KP, Elting L, et al. Use of Radiation Therapy in the Last 30 Days of Life Among a Large Population-Based Cohort of Elderly Patients in the United States. J Clin Oncol 2013;31:80-7. [Crossref] [PubMed]
- Chow E, Abdolell M, Panzarella T, et al. Predictive model for survival in patients with advanced cancer. J Clin Oncol 2008;26:5863-9. [Crossref] [PubMed]
- Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: The TEACHH model. Cancer 2014;120:134-41. [Crossref] [PubMed]
- Zucker A, Tsai CJ, Loscalzo J, et al. The NEAT Predictive Model for Survival in Patients with Advanced Cancer. Cancer Res Treat 2018;50:1433-43. [PubMed]
- Chow E, Abdolell M, Panzarella T, et al. Validation of a Predictive Model for Survival in Metastatic Cancer Patients Attending an Outpatient Palliative Radiotherapy Clinic. Int J Radiat Oncol Biol Phys 2009;73:280-87. [Crossref] [PubMed]
- Zucker A, Tsai CJ, Loscalzo J, et al. The NEAT Predictive Model for Survival in Patients with Advanced Cancer. Cancer Res Treat 2018;50:1433-43. [Crossref] [PubMed]
- Dosani M, Tyldesley S, Bakos B, et al. The TEACHH model to predict life expectancy in patients presenting for palliative spine radiotherapy: external validation and comparison with alternate models. Support Care Cancer 2018;26:2217-27. [Crossref] [PubMed]
- Glare P, Shariff I, Thaler HT. External Validation of the Number of Risk Factors Score in a Palliative Care Outpatient Clinic at a Comprehensive Cancer Center. J Palliat Med 2014;17:797-802. [Crossref] [PubMed]
- Gwilliam B, Keeley V, Todd C, et al. Development of Prognosis in Palliative care Study (PiPS) predictor models to improve prognostication in advanced cancer: prospective cohort study. BMJ 2011;343:d4920. [Crossref] [PubMed]
- Gilligan T, Coyle N, Frankel RM, et al. Patient-Clinician Communication: American Society of Clinical Oncology Consensus Guideline. J Clin Oncol 2017;35:3618-32. [Crossref] [PubMed]
- Weeks JC, Catalano PJ, Cronin A, et al. Patients' Expectations about Effects of Chemotherapy for Advanced Cancer. N Engl J Med 2012;367:1616-25. [Crossref] [PubMed]
- Chen AB, Cronin A, Weeks JC, et al. Expectations About the Effectiveness of Radiation Therapy Among Patients With Incurable Lung Cancer. J Clin Oncol 2013;31:2730-5. [Crossref] [PubMed]
- Enzinger AC, Zhang B, Schrag D, et al. Outcomes of Prognostic Disclosure: Associations With Prognostic Understanding, Distress, and Relationship With Physician Among Patients With Advanced Cancer. J Clin Oncol 2015;33:3809-16. [Crossref] [PubMed]
- Mack JW, Weeks JC, Wright AA, et al. End-of-Life Discussions, Goal Attainment, and Distress at the End of Life: Predictors and Outcomes of Receipt of Care Consistent With Preferences. J Clin Oncol 2010;28:1203-08. [Crossref] [PubMed]
- Kirk P, Kirk I, Kristjanson LJ. What do patients receiving palliative care for cancer and their families want to be told? A Canadian and Australian qualitative study. BMJ 2004;328:1343. [Crossref] [PubMed]
- Apatira L, Boyd EA, Malvar G, et al. Hope, truth, and preparing for death: Perspectives of surrogate decision makers. Ann Intern Med 2008;149:861-8. [Crossref] [PubMed]
- Tanco K, Rhondali W, Perez-Cruz P, et al. Patient perception of physician compassion after a more optimistic vs a less optimistic message: A randomized clinical trial. JAMA Oncol 2015;1:176-83. [Crossref] [PubMed]
- Jenkins V, Fallowfield L, Saul J. Information needs of patients with cancer: results from a large study in UK cancer centres. Br J Cancer 2001;84:48. [Crossref] [PubMed]
- Hagerty RG, Butow PN, Ellis PM, et al. Communicating prognosis in cancer care: a systematic review of the literature. Ann Oncol 2005;16:1005-53. [Crossref] [PubMed]
- Mack JW, Fasciano KM, Block SD. Communication About Prognosis With Adolescent and Young Adult Patients With Cancer: Information Needs, Prognostic Awareness, and Outcomes of Disclosure. J Clin Oncol 2018;36:1861-7. [Crossref] [PubMed]
- Oostendorp LJ, Ottevanger PB, van de Wouw AJ, et al. Patients’ Preferences for Information About the Benefits and Risks of Second-Line Palliative Chemotherapy and Their Oncologist’s Awareness of These Preferences. J Cancer Educ 2016;31:443-8. [Crossref] [PubMed]
- Gattellari M, Voigt KJ, Butow PN, et al. When the treatment goal is not cure: are cancer patients equipped to make informed decisions? J Clin Oncol 2002;20:503-13. [Crossref] [PubMed]
- Marron JM, Cronin Angel M, Kang Tammy I, et al. Intended and unintended consequences: Ethics, communication, and prognostic disclosure in pediatric oncology. Cancer 2018;124:1232-41. [Crossref] [PubMed]
- Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665-73. [Crossref] [PubMed]
- Bernacki RE, Block SD. for the American College of Physicians High Value Care Task F. Communication about serious illness care goals: A review and synthesis of best practices. JAMA Intern Med 2014;174:1994-2003. [Crossref] [PubMed]
- Baile WF, Buckman R, Lenzi R, et al. SPIKES—A Six-Step Protocol for Delivering Bad News: Application to the Patient with Cancer. Oncologist 2000;5:302-11. [Crossref] [PubMed]
- Umezawa S, Fujimori M, Matsushima E, et al. Preferences of advanced cancer patients for communication on anticancer treatment cessation and the transition to palliative care. Cancer 2015;121:4240-9. [Crossref] [PubMed]
- Abdul-Razzak A, You J, Sherifali D, et al. ‘Conditional candour’ and ‘knowing me’: an interpretive description study on patient preferences for physician behaviours during end-of-life communication. BMJ Open 2014;4:e005653. [Crossref] [PubMed]
- Clayton JM, Hancock K, Parker S, et al. Sustaining hope when communicating with terminally ill patients and their families: a systematic review. Psychooncology 2008;17:641-59. [Crossref] [PubMed]
- Lutz S, Chow E, Hoskin P. Radiation Oncology in Palliative Cancer Care. First edition. Hoboken, New Jersey: Wiley-Blackwell, 2013.
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [Crossref] [PubMed]
- Blum RH, Novetsky D, Shasha D, et al. The multidisciplinary approach to bone metastases. Oncology (Williston Park) 2003;17:845-57; discussion 62-3, 67.
- Chow E, Zeng L, Salvo N, et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112-24. [Crossref] [PubMed]
- Wu JS, Wong RK, Lloyd NS, et al. Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases - an evidence-based practice guideline. BMC Cancer 2004;4:71. [Crossref] [PubMed]
- Kaasa S, Brenne E, Lund JA, et al. Prospective randomised multicenter trial on single fraction radiotherapy (8 Gy x 1) versus multiple fractions (3 Gy x 10) in the treatment of painful bone metastases. Radiother Oncol 2006;79:278-84. [Crossref] [PubMed]
- Roos DE, Turner SL, O'Brien PC, et al. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05). Radiother Oncol 2005;75:54-63. [Crossref] [PubMed]
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101-9. [Crossref] [PubMed]
- William F. Randomized Trial of Short- Versus Long-Course Radiotherapy for Palliation of Painful Bone Metastases. J Natl Cancer Inst 2005;97:798-804. [Crossref] [PubMed]
- Kachnic L, Berk L. Palliative single-fraction radiation therapy: how much more evidence is needed? J Natl Cancer Inst 2005;97:786-8. [Crossref] [PubMed]
- Chow E, van der Linden YM, Roos D, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol 2014;15:164-71. [Crossref] [PubMed]
- Meeuse JJ, van der Linden YM, van Tienhoven G, et al. Efficacy of radiotherapy for painful bone metastases during the last 12 weeks of life: results from the Dutch Bone Metastasis Study. Cancer 2010;116:2716-25. [PubMed]
- Spencer K, Parrish R, Barton R, et al. Palliative radiotherapy. BMJ 2018;360:k821. [Crossref] [PubMed]
- Hird A, Chow E, Zhang L, et al. Determining the Incidence of Pain Flare Following Palliative Radiotherapy for Symptomatic Bone Metastases: Results From Three Canadian Cancer Centers. Int J Radiat Oncol Biol Phys 2009;75:193-7. [Crossref] [PubMed]
- Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer 2013;119:888-96. [Crossref] [PubMed]
- Katagiri H, Takahashi M, Inagaki J, et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys 1998;42:1127-32. [Crossref] [PubMed]
- Nieder C, Grosu AL, Andratschke NH, et al. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys 2006;66:1446-9. [Crossref] [PubMed]
- Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria (R) spinal bone metastases. J Palliat Med 2013;16:9-19. [Crossref] [PubMed]
- Lipton A, Fizazi K, Stopeck AT, et al. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur J Cancer 2016;53:75-83. [Crossref] [PubMed]
- Orcajo-Rincon J, Caresia-Aroztegui AP, Del Puig Cozar-Santiago M, et al. Radium-223 in the treatment of bone metastasis in patients with castration-resistant prostate cancer. Review and procedure. Rev Esp Med Nucl Imagen Mol 2018;37:330-7. [Crossref] [PubMed]
- Fehlings MG, Nater A, Tetreault L, et al. Survival and Clinical Outcomes in Surgically Treated Patients With Metastatic Epidural Spinal Cord Compression: Results of the Prospective Multicenter AOSpine Study. J Clin Oncol 2016;34:268-76. [Crossref] [PubMed]
- Lam TC, Uno H, Krishnan M, et al. Adverse Outcomes After Palliative Radiation Therapy for Uncomplicated Spine Metastases: Role of Spinal Instability and Single-Fraction Radiation Therapy. Int J Radiat Oncol Biol Phys 2015;93:373-81. [Crossref] [PubMed]
- Koswig S, Budach V. Remineralization and pain relief in bone metastases after after different radiotherapy fractions (10 times 3 Gy vs. 1 time 8 Gy). A prospective study. Strahlenther Onkol 1999;175:500-8. [Crossref] [PubMed]
- Huisman M, van der Velden JM, van Vulpen M, et al. Spinal instability as defined by the Spinal Instability Neoplastic Score (SINS) is associated with radiotherapy failure in metastatic spinal disease. Spine J 2014;14:2835-40. [Crossref] [PubMed]
- Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221-9. [Crossref] [PubMed]
- Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol 2011;29:3072-7. [Crossref] [PubMed]
- Fisher CG, Schouten R, Versteeg AL, et al. Reliability of the Spinal Instability Neoplastic Score (SINS) among radiation oncologists: an assessment of instability secondary to spinal metastases. Radiat Oncol 2014;9:69. [Crossref] [PubMed]
- Versteeg AL, van der Velden JM, Verkooijen HM, et al. The Effect of Introducing the Spinal Instability Neoplastic Score in Routine Clinical Practice for Patients With Spinal Metastases. Oncologist 2016;21:95-101. [Crossref] [PubMed]
- Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 2011;12:225-35. [Crossref] [PubMed]
- Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 2010;76:S42-9. [Crossref] [PubMed]
- Rades D, Stalpers LJ, Veninga T, et al. Spinal reirradiation after short-course RT for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 2005;63:872-5. [Crossref] [PubMed]
- Cheney M, Lam T-C, Uno H, et al. Outcomes after palliative re-irradiation of spinal metastases. J Radiat Oncol 2015;4:157-62. [Crossref]
- Bhattacharya IS, Hoskin PJ. Stereotactic body radiotherapy for spinal and bone metastases. Clin Oncol (R Coll Radiol) 2015;27:298-306. [Crossref] [PubMed]
- Hong LX, Shankar V, Shen J, et al. Spine stereotactic body radiation therapy plans: Achieving dose coverage, conformity, and dose falloff. Med Dosim 2015;40:181-5. [Crossref] [PubMed]
- Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:107-16. [Crossref] [PubMed]
- Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila Pa 1976) 2009;34:S78-92. [Crossref] [PubMed]
- Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys 2008;71:652-65. [Crossref] [PubMed]
- Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 Phase II/III Study of Image-Guided Stereotactic Radiosurgery for Localized (1-3) Spine Metastases: Phase II Results. Int J Radiat Oncol Biol Phys 2011;81:S131-2. [Crossref] [PubMed]
- Sprave T, Verma V, Forster R, et al. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol 2018;128:274-82. [Crossref] [PubMed]
- Haley ML, Gerszten PC, Heron DE, et al. Efficacy and cost-effectiveness analysis of external beam and stereotactic body radiation therapy in the treatment of spine metastases: a matched-pair analysis. J Neurosurg Spine 2011;14:537-42. [Crossref] [PubMed]
- Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist 2013;18:744-51. [Crossref] [PubMed]
- Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine 2010;13:324-8. [Crossref] [PubMed]
- Quraishi NA, Arealis G, Salem KM, et al. The surgical management of metastatic spinal tumors based on an Epidural Spinal Cord Compression (ESCC) scale. Spine J 2015;15:1738-43. [Crossref] [PubMed]
- Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 1969;7:179-92. [PubMed]
- Curtin M, Piggott RP, Murphy EP, et al. Spinal Metastatic Disease: A Review of the Role of the Multidisciplinary Team. Orthop Surg 2017;9:145-51. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Rades D, Segedin B, Conde-Moreno AJ, et al. Radiotherapy With 4 Gy x 5 Versus 3 Gy x 10 for Metastatic Epidural Spinal Cord Compression: Final Results of the SCORE-2 Trial (ARO 2009/01). J Clin Oncol 2016;34:597-602. [Crossref] [PubMed]
- Hoskin P, Misra V, Hopkins K, et al. SCORAD III: Randomized noninferiority phase III trial of single-dose radiotherapy (RT) compared to multifraction RT in patients (pts) with metastatic spinal canal compression (SCC). J Clin Oncol 2017;35:LBA10004. [Crossref]
- Sahgal A, Bilsky M, Chang EL, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine 2011;14:151-66. [Crossref] [PubMed]
- Moussazadeh N, Laufer I, Yamada Y, et al. Separation surgery for spinal metastases: effect of spinal radiosurgery on surgical treatment goals. Cancer Control 2014;21:168-74. [Crossref] [PubMed]
- Soffietti R, Cornu P, Delattre JY, et al. Brain Metastases. In: Gilhus NE, Barnes MP, Brainin M, editor. European Handbook of Neurological Management. Second edition. Hoboken: Wiley-Blackwell, 2010:437-45.
- Sperduto PW, Yang TJ, Beal K, et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol 2017;3:827-31. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Soffietti R, Cornu P, Delattre JY, et al. EFNS Guidelines on diagnosis and treatment of brain metastases: report of an EFNS Task Force. Eur J Neurol 2006;13:674-81. [Crossref] [PubMed]
- Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys 2009;75:1125-31. [Crossref] [PubMed]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013;31:65-72. [Crossref] [PubMed]
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004-14. [Crossref] [PubMed]
- Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012;2:210-25. [Crossref] [PubMed]
- NCCN. Central Nerous System Cancers. In: Network NCC, ed. NCCN Clinical Practice Guidelines in Oncology, 2013.
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Brown PD, Ballman KV, Cerhan J, et al. N107C/CEC.3: A Phase III Trial of Post-Operative Stereotactic Radiosurgery (SRS) Compared with Whole Brain Radiotherapy (WBRT) for Resected Metastatic Brain Disease. Int J Radiat Oncol Biol Phys 2016;96:937. [Crossref]
- Mahajan A, Ahmed S, Li J, et al. Postoperative Stereotactic Radiosurgery Versus Observation for Completely Resected Brain Metastases: Results of a Prospective Randomized Study. Int J Radiat Oncol Biol Phys 2016;96:S2. [Crossref]
- Feuvret L, Vinchon S, Martin V, et al. Stereotactic radiotherapy for large solitary brain metastases. Cancer Radiother 2014;18:97-106. [Crossref] [PubMed]
- Inoue HK, Sato H, Seto KI, et al. Five-fraction CyberKnife radiotherapy for large brain metastases in critical areas: impact on the surrounding brain volumes circumscribed with a single dose equivalent of 14 Gy (V14) to avoid radiation necrosis. J Radiat Res 2014;55:334-42. [Crossref] [PubMed]
- Gondi V, Hermann BP, Mehta MP, et al. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys 2012;83:e487-93. [Crossref] [PubMed]
- Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014;32:3810-6. [Crossref] [PubMed]
- Gondi V, Mehta MP, Pugh S, et al. Memory Preservation With Conformal Avoidance of the Hippocampus During Whole-Brain Radiation Therapy for Patients With Brain Metastases: Primary Endpoint Results of RTOG 0933. Int J Radiat Oncol Biol Phys 2013;87:1186. [Crossref]
- Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429-37. [Crossref] [PubMed]
- Rodrigues G, Movsas B. Palliative radiotherapy in advanced lung cancer. In: Lutz S, Chow E, Hoskin P, editor. Radiation Oncology in Palliativ Cancer Care. Hoboken: Wiley-Blackwell 2013:163-76.
- Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol 2008;26:4001-11. [Crossref] [PubMed]
- A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer 1992;65:934-41. [Crossref] [PubMed]
- Macbeth FR, Bolger JJ, Hopwood P, et al. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:167-75. [Crossref] [PubMed]
- Lutz ST, Jones J, Chow E. Role of Radiation Therapy in Palliative Care of the Patient With Cancer. J Clin Oncol 2014;32:2913-9. [Crossref] [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. The use of self-expanding stents in esophageal and gastroesophageal junction cancer palliation: a meta-analysis and meta-regression analysis of outcomes. Dig Dis Sci 2010;55:3018-30. [Crossref] [PubMed]
- Leslie MD, Dische S, Saunders MI, et al. The role of radiotherapy in carcinoma of the thoracic oesophagus: an audit of the Mount Vernon experience 1980-1989. Clin Oncol (R Coll Radiol) 1992;4:114-8. [Crossref] [PubMed]
- Freilich J, Hoffe SE, Almhanna K, et al. Comparative outcomes for three-dimensional conformal versus intensity-modulated radiation therapy for esophageal cancer. Dis Esophagus 2015;28:352-7. [Crossref] [PubMed]
- Lee YH, Lee JW, Jang HS. Palliative external beam radiotherapy for the treatment of tumor bleeding in inoperable advanced gastric cancer. BMC Cancer 2017;17:541. [Crossref] [PubMed]
- Tey J, Choo BA, Leong CN, et al. Clinical outcome of palliative radiotherapy for locally advanced symptomatic gastric cancer in the modern era. Medicine (Baltimore) 2014;93:e118. [Crossref] [PubMed]
- Rominger CJ, Gunderson LL, Gelber RD, et al. Radiation therapy alone or in combination with chemotherapy in the treatment of residual or inoperable carcinoma of the rectum and rectosigmoid or pelvic recurrence following colorectal surgery. Radiation Therapy Oncology Group study (76-16). Am J Clin Oncol 1985;8:118-27. [Crossref] [PubMed]
- Bae SH, Park W, Choi DH, et al. Palliative radiotherapy in patients with a symptomatic pelvic mass of metastatic colorectal cancer. Radiat Oncol 2011;6:52. [Crossref] [PubMed]
- Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol 2013;8:58. [Crossref] [PubMed]
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9. [Crossref] [PubMed]
- Hui D, Shamieh O, Paiva CE, et al. Minimal Clinically Important Difference in the Physical, Emotional, and Total Symptom Distress Scores of the Edmonton Symptom Assessment System. J Pain Symptom Manage 2016;51:262-9. [Crossref] [PubMed]
- Hui D, Titus A, Curtis T, et al. Implementation of the Edmonton Symptom Assessment System for Symptom Distress Screening at a Community Cancer Center: A Pilot Program. Oncologist 2017;22:995-1001. [Crossref] [PubMed]
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain 2008;24:497-508. [Crossref] [PubMed]
- Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA 1984;252:1905-7. [Crossref] [PubMed]
- Finkelman MD, Kulich RJ, Zacharoff KL, et al. Shortening the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R): A Proof-of-Principle Study for Customized Computer-Based Testing. Pain Med 2015;16:2344-56. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. [Crossref] [PubMed]
- Bickel KE, McNiff K, Buss MK, et al. Defining High-Quality Palliative Care in Oncology Practice: An American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine Guidance Statement. J Oncol Pract 2016;12:e828-38. [Crossref] [PubMed]
- Puchalski CM. Spirituality in the cancer trajectory. Ann Oncol 2012;23 Suppl 3:49-55. [Crossref] [PubMed]
- Puchalski C. Spiritual assessment in clinical practice. Ann Psychiatry 2006;36:150.
- Borneman T, Ferrell B, Puchalski CM. Evaluation of the FICA Tool for Spiritual Assessment. J Pain Symptom Manage 2010;40:163-73. [Crossref] [PubMed]
- Anandarajah G, Hight E. Spirituality and medical practice: using the HOPE questions as a practical tool for spiritual assessment. Am Fam Physician 2001;63:81-9. [PubMed]
- Maugans TA. The SPIRITual history. Arch Fam Med 1996;5:11-6. [Crossref] [PubMed]