
What are the criteria for response to cachexia treatment?
Introduction
Currently there are no agents approved by the Food and Drug Administration (FDA) for the treatment of cancer cachexia. While there are many factors that contribute to this, certainly part of the issue has been lack of consensus on what the proper endpoints might be for clinical trials and the need for regulatory guidance to help us move the field forward (1). As a result, promising agents have fallen short of approval in large phase III trials. Despite the huge unmet medical need, investment in this field of study has suffered by a lack of a clear pathway to approval.
One of the barriers to the study of cachexia was the lack of a commonly accepted definition until the International Consensus Conference, led by Ken Fearon (2). This international consensus group developed the following definition: “Cancer cachexia is defined as a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment. Its pathophysiology is characterized by negative protein and energy balance driven by a variable combination of reduced food intake and abnormal metabolism”.
This definition has been widely accepted in the cachexia community and has been quite helpful in focusing attention on common aspects of the syndrome, including muscle loss and change in body composition, impaired nutrition and negative energy balance, and decline in physical function. For the successful treatment of cachexia, criteria of response must address all three of these domains.
Body composition
The clinical diagnostic criteria for cachexia include weight loss of >5% or a body mass index (BMI) of <20 kg/m2 with 2% weight loss. The importance of cancer associated weight loss was demonstrated in a large study of over 8,000 Canadian and European patients with cancer, 89% of whom had locally advanced or metastatic disease. BMI and percent weight loss data were collected, in addition to information on disease, stage, performance status and other variables. Patients were followed for survival (3). As outlined in Figure 1, there is a clear relationship between weight loss and reduced survival. For example, patients with less than 2.5% weight loss had an average median survival of 17.3 months, and those who had lost more than 15% of their weight loss had a median survival of 4.4 months. Of note, body composition as measured by BMI (kg/m2) was also related to decrease in survival with patients above a BMI of 28, having a survival of 13.1 months, while patients below a BMI of 20 had a median survival of 4.7 months. As noted in the figure, there is a clear interaction between BMI and weight loss demonstrating that both measures are extremely important to outcome.
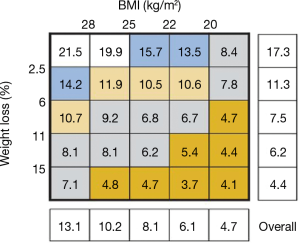
With weight loss as an essential component of cachexia, any successful therapeutic intervention would need to demonstrate reversal of weight loss and corresponding change in BMI. However, baseline BMI alone may not be adequate as an overall measurement of body composition. For example, in analysis of clinical outcomes, patients with advanced non-small cell lung cancer (NSCLC) enrolled on Eastern Cooperative Oncology Group clinical trials, a low body BMI was associated with a shorter survival compared to normal, but patients with a high BMI actually had a better survival compared to normal (4). In a retrospective observational study of patients with stages I–III colorectal cancer undergoing surgery, patients with a low BMI again had an increased hazard ratio for overall mortality, but an even more dramatic increase in overall mortality was seen in the obese population (5).
Thus, obesity and high BMI may have varying association with outcome depending on the disease state. In addition to understanding the important relationship of weight loss to BMI, it is important to understand the components of body composition included in BMI. The pioneering work of Kazemi-Bajestani and colleagues utilizing computed tomography to define muscle and fat wasting is critical to a more refined understanding of cachexia and potential measures that can be used to assess response to cachexia treatment (6). These investigators developed software that can be applied to standard cross-sectional CT imaging to quantitatively evaluate muscle and visceral, subcutaneous and intramuscular fat in CT scans. Their work and others have clearly demonstrated that the central issue in serial follow up of advanced cancer patients is the ongoing depletion of muscle (sarcopenia) that occurs. This loss of muscle mass is associated with increased toxicity from chemotherapy, prolonged length of hospital stay and postoperative complications after cancer surgery, and shorter survival.
Moreover, in the current epidemic of obesity, skeletal muscle depletion is a powerful prognostic factor which is independent of BMI (7). In this study, over 1,400 patients with lung or GI cancer were assessed at presentation for weight loss with measurement of lumbar skeletal muscle index (SMI) and mean muscle attenuation by computed tomography. Each of these variables were found to be prognostic for survival. For patients who had all 3 of these core prognostic variables, survival is 8.4 months, regardless of the patient’s BMI and in contrast, the patient who had none of these features, the survival was in excess of 28 months.
The importance of sarcopenia to outcome is not limited to advanced cancer patients. A study of over 3,000 women with stage II or III breast cancer were assessed by CT scan for the presence of sarcopenia, poor muscle quality and excess adiposity derived from clinically obtained CT scans (8). Among women with non-metastatic breast cancer, those with sarcopenia showed a higher overall mortality with a hazard ratio of 1.41. Likewise, patients at the highest tertiles of total adipose tissue also showed a higher overall mortality of 1.35. The highest mortality was seen in patients with both sarcopenia and a high total adiposity score. These patients with sarcopenic obesity had a hazard ratio for mortality of 1.89. BMI alone was not significantly related to overall mortality and did not identify patients at risk.
Part of the relationship of body composition and outcome may be explained by response to neoadjuvant chemotherapy (9). Patients with a pathologic complete response to neoadjuvant chemotherapy were matched with controls who did not achieve a pathologic complete response, and body composition was analyzed between the two groups by computed tomography imaging. Overweight and obese patients treated with neoadjuvant chemotherapy had a lower pathologic complete response rate and shorter progression free survival time. Among patients with a normal BMI, the pathologic complete response rate was actually better in the sarcopenic group. This suggests that skeletal muscle is a major contributor to the pharmacokinetics and pharmacodynamics of chemotherapy. It also explains why body composition per se is such a powerful predictor of toxicity.
In a study of 151 patients with early stage breast cancer, body composition metrics were determined by pretreatment computerized tomographic images in relationship to subsequent toxicity from anthracycline and taxane based chemotherapy (10). One third of patients developed grade 3/4 toxicity which was significantly higher in those with a low lean body mass (LBM) (relative rate 1.48). A low skeletal muscle gauge was calculated as the SMI divided by height squared times the skeletal muscle density. After adjusting for age and body surface area, a low skeletal muscle gauge was significantly associated with hematologic and gastrointestinal grade 3 and 4 toxicities, as well as hospitalization.
Thus, body composition can be an important determinant of likelihood of response to treatment as well as toxicity and suggests that body composition could be useful to develop better dosing paradigms for chemotherapy in different populations.
In addition to the importance of baseline skeletal muscle mass in predicting response and toxicity from chemotherapy in cancer patients, changes in skeletal muscle occur as a dynamic process during cancer treatment (11). In a series of patients receiving palliative chemotherapy with advanced lung cancer, almost half of the patients had a stable or increased muscle mass during chemotherapy. Nearly all of these patients had a tumor response to the chemotherapy, while the other patients with disease progression had ongoing skeletal muscle loss. The increase in muscle mass turned out to be a significant prognostic factor, compared to baseline sarcopenia alone, which wasn’t prognostic in this study.
The complex relationship between obesity, sarcopenia and cachexia was studied in a prospective evaluation of 200 patients with lung cancer (12). Routine staging CT was done before and after chemotherapy and a variety of body composition measures were determined. In general, increases were seen in a number of measures of adiposity while there were decreases in muscle area and muscle density measures after chemotherapy. These measures were not adequately predicted by BMI or weight loss alone, demonstrating the importance of the use of body composition analysis prior to and during treatment of cancer patients.
It is also important to put body composition in the context of aging. Muscle quality or strength mass ratio declines with aging, but it is highly variable across individuals (13). In a study, over 500 healthy men and women age 50 years and older were followed for an average of 4 years. Muscle quality showed a significant decline over time. Neither baseline weight or BMI were predictive of this decline, but higher total body fat mass and lower total lean mass at baseline predicted for a steeper decline in muscle quality over time. The conclusion of this study was that preventive strategies and the maintaining of muscle quality during aging should not focus on weight or BMI per se, but specifically target body composition features.
Summary of body composition measures
Cancer cachexia is defined by weight loss. Any criteria to assess treatment of cachexia must include a measure of reversal of the weight loss process. However, it is equally clear that measurement of weight and BMI alone are not sufficient parameters to assess response to treatment. Body composition has emerged as the most important measure to determine patients with or at risk of developing cachexia and muscle loss. Equally important is the need to be able to monitor patients over time to evaluate the impact of cancer, as well as cancer treatment on body composition. While DEXA scans and other applications can be considered for monitoring outcomes of patients with cancer cachexia, the availability of CT imaging and its standardization and assessment of tumor response along with the development of software that allows precise characterization of skeletal muscle and fat mass provides an ideal tool that can be incorporated not only in clinical investigation, but potentially also in standard clinical practice.
Nutritional endpoints
The pathophysiology of cancer cachexia is characterized by a negative protein and energy balance and driven by abnormal metabolism (14). A significant component of the negative energy balance is related to decreased intake of food secondary to anorexia that may be associated with cancer per se or cancer treatment. In addition, physical factors may influence swallowing, such as patients with head and neck cancer receiving radiation treatment, for example. However, an important component in nutritional depletion of the cancer patient may be related to altered metabolism that may coexist with anorexia and reduced food intake.
The metabolic pathways that impact metabolism in cancer cachexia are complex (15). Mediators of cachexia include TNF-alpha, interleukin 6 and other inflammatory mediators. In addition, myostatin and activin play an important role in muscle loss. In the setting of cancer, neuroendocrine changes occur as a stress response with changes in the pituitary adrenal axis and reduced insulin sensitivity among others. A net effect is the state of hyper-metabolism with total energy expenditure leading to a negative energy balance.
In such a complicated clinical environment, it is difficult to identify a solitary factor that would be a useful measure of response to therapy for cachexia. Biomarkers, while quite useful in understanding some of the pathophysiology of cachexia, they are less well established for clinical use (16). Of all the inflammatory markers, C-reactive protein has been widely studied in cachexia and is of prognostic significance, but has not been shown to be useful in identifying and monitoring patients with cancer cachexia. Numerous other biomarkers including parathyroid hormone related protein, insulin, cortisol and interleukin levels have been measured in laboratory and clinical studies of cachexia. But again, due to the complexity of the interaction of these cytokines and neuroendocrine signals, none of these have been established as a biomarker for detection or monitoring of patients with cachexia.
Another approach to metabolic assessment is resting energy expenditure which has been measured prospectively in cancer patients (17). In a study of 390 patients, nearly 50% were shown to be hypermetabolic. Compared to patients with normal metabolism, the hypermetabolic group were more likely to have a negative energy balance, weight loss >5%, performance status of 2 or greater and elevated CRP concentrations. In patients with metastatic disease, those with hypermetabolism had a reduced median survival compared to those with normal metabolism (14.6 vs. 21.4 months). Resting energy expenditure is the amount of energy that is expended in 24 hours by the body at rest and can be measured by direct or indirect calorimetry. The identification of patients with hypermetabolism may be useful in targeting nutritional interventions.
Although by definition cachexia cannot be fully reversed by nutritional support, serial studies of CT imaging in cancer patients have identified a window of anabolic potential early in the disease trajectory where there may be an opportunity for nutritional intervention to stop or reverse cachexia (18). Guidelines supporting nutritional screening and specific intervention to reduce malnutrition and the loss of muscle mass have been well studied (19). Stepwise nutritional interventions are recommended in screening and monitoring of patients with cancer to assess energy and substrate requirements. Of note, it is recommended clinically that nutritional care should be accompanied with exercise training. Further study in this area is needed, since despite a strong rationale for the use of exercise, a Cochrane systematic review found insufficient evidence in the safety and effectiveness of exercise in cancer cachexia patients (20).
A study to better understand this complex multifaceted syndrome of cancer cachexia is the MENAC trial (multimodal intervention in advanced cancer patients undergoing chemotherapy; NCT02330926) (21). This trial is looking at nutritional supplementation and advice, a home based self-assisted exercise program and anti-inflammatory medication to treat cachexia. A simple prospective clinical trial, the primary endpoint is to increase the body weight as described previously. While body composition may be a superior tool to understand the impact of cachexia, and therapeutic interventions in cachexia, body weight remains an important clinical endpoint for patients and family members.
Summary of nutritional endpoints
To better understand the multifactorial syndrome of cachexia, measurements of a variety of cytokines and other potential mediators for cachexia, along with sophisticated measures of energy expenditure are important investigative tools. However, in monitoring response to therapeutic interventions for cachexia, whether pharmacologic or non-pharmacologic approaches are used, simple response endpoints of nutritional assessment of intake and body weight remain valuable and valid clinical endpoints.
Progressive functional impairment
The measurement of performance status in cancer patients is a time-honored tradition in oncology that has been well validated and a prognostic tool for overall survival (22). The performance status score is in fact a measure of functional decline that occurs as an inevitable part of the cachexia syndrome. Therefore, an important part of measuring response to therapeutic intervention for cachexia would be to show a reversal of that functional decline. Unfortunately that has been a very difficult task to date and certainly a major barrier to the approval of agents for cachexia by regulatory authorities.
While the relationship between muscle mass and muscle strength has been established, it is not clear whether higher muscle mass translates directly into greater muscle strength or whether increases in muscle strength lead to increase in muscle mass. In a population-based study of over 2,600 older individuals in the United States, there was a positive correlation between muscle mass and muscle strength. This correlation was independent of associations of age and gender with muscle mass and strength (23). It was noted that comorbid medical conditions are independent predictors of lower muscle strength in this population, including diabetes, heart failure, kidney disease and obesity, all of which modify the relationship between muscle mass and muscle strength. In another study of older adults with diabetes, arm and leg muscle mass were greater in patients with diabetes, but the muscle strength was lower than their corresponding counterparts (24). These differences were further impacted by longer duration of diabetes and poor glycemic control.
In a study of 1,500 hospitalized patients, assessed for weight loss, both men and women exhibited a stepwise decrease in hand grip strength with increasing weight loss. However, for patients with severe weight loss, further reduction of hand grip strength was significantly greater in men than women, emphasizing a dimorphic effect of cachexia on muscle strength (25).
The relationship between muscle mass and muscle function can be further complicated by measurements being used. In a study of 241 advanced cancer patients, cachexia was assessed by weight loss and BMI and muscle mass was determined by 3 different measurements, upper arm muscle area, computed tomography and bio-electrical impedance analysis. In addition, measurement of appetite, inflammation, muscle strength by hand grip, fatigue, quality of life and survival were all measured. All 3 measures of muscle differed in terms of percentage of patients who met criteria for cachexia by these studies, including variability in markers of inflammation and hand grip strength (26). With the impact of sex, comorbidity and different measurement techniques on muscle and muscle function, it is not surprising that it might be difficult to identify a gold standard for a functional measure of response to a therapeutic agent in cachexia.
In the ROMANA 1 and 2 trials, anamorelin, the ghrelin agonist was studied in randomized double-blind placebo-controlled trials in patients with advanced NSCLC and cachexia (27). Co-primary endpoints included median change in LBM and hand grip strength over 12 weeks. Despite significant increases in LBM in the anamorelin group compared to a decrease in the placebo group, no differences were seen in hand grip strength, which declined in both populations in both studies.
In the study of the selective androgen receptor modulator, enobosarm, in patients with advanced lung cancer undergoing initial platinum-based chemotherapy, co-primary endpoints were again chosen, but this time included LBM along with stair climb power as a measure of physical function (28). In these trials, clear association between enobosarm and improvement in LBM in the treatment group versus decline in LBM in the placebo group was demonstrated. However, there was an inconsistent effect on stair climb power between the two studies (29). Despite the improvement in LBM with both anamorelin and enobosarm, the lack of a correlation with a functional test led to the regulatory interpretation that these are negative trials. At present, neither anamorelin nor the enobosarm are approved for clinical use.
Another potential criteria for clinical benefit of agents in cachexia include patient reported outcomes. In the ROMANO trials, anamorelin was associated with a clear improvement in appetite as measured by the anorexia/cachexia scale as a functional assessment of anorexia/cachexia therapy (FAACT) (27). Of note, both trials also demonstrated that the anamorelin group had an average increase in weight from baseline of 1–2 kgs, while the control group showed a decline in body weight, thus correlating a patient reported outcome with improvement in weight and LBM. Despite confirmatory trials in Japan with these endpoints, approval of this agent is still pending (30).
The pathway forward for incorporation of functional measures of improvement of cachexia treatment is unclear. A promising agent such as anamorelin that reverses muscle loss associated with positive effects on patient reported outcomes of anorexia and increase in body weight would be viewed as clinically beneficial by both patients and providers. Other promising approaches are under study (31). Physical measurements used in clinical trials such as hand grip strength, stair climb power, 6-minute walk or other functional tests may be difficult to administer depending on the patient and may or may not be clinically meaningful endpoints. Actigraphy as well as patient reported outcomes that measure physical and functional activity are of interest, but these have not been formally tested in prospective phase III cachexia interventional studies.
Summary
The management of cancer cachexia remains a major unmet medical need. Even in its earliest stages, prior to clinical recognition, cachexia/muscle loss can impact response and toxicity from cancer treatment. As cachexia advances over time, there is associated decline in physical function and reduced survival. Measurements of response in cachexia interventional trials should continue to focus on reversal of weight loss and improvements in body composition, specifically LBM. Rather than focus on specific functional tasks, improvement in patient reported outcomes may be the most appropriate measure of clinical improvement. Due to the multifactorial nature of cachexia, multimodal interventional trials such as the MENAC study, provide the best opportunity to correlate response with a therapeutic intervention.
Acknowledgements
I would like to acknowledge Teressa Green for her administrative assistance with the manuscript.
Footnote
Conflicts of Interest: The author declares Consultant/Independent Contract: Amgen, AstraZeneca, Coherus, Enzychem, Merck, Pfizer; Grant/Research Support: AstraZeneca, Genentech, Helsinn; Chair/DSMB Member: Beyond Spring, G1 Therapeutics, Janssen, Merrimack, Mylan, Roche.
References
- Fearon K, Argiles JM, Baracos VE, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle 2015;6:272-4. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Martin L, Senesse P, Gioulbasanis I, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol 2015;33:90-9. [Crossref] [PubMed]
- Dahlberg SE, Schiller JH, Bonomi PB, et al. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol 2013;8:1121-7. [Crossref] [PubMed]
- Kroenke CH, Neugebauer R, Meyerhardt J, et al. Analysis of Body Mass Index and Mortality in Patients With Colorectal Cancer Using Causal Diagrams. JAMA Oncol 2016;2:1137-45. [Crossref] [PubMed]
- Kazemi-Bajestani SM, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 2016;54:2-10. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol 2018;4:798-804. [Crossref] [PubMed]
- Del Fabbro E, Parsons H, Warneke CL, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist 2012;17:1240-5. [Crossref] [PubMed]
- Shachar SS, Deal AM, Weinberg M, et al. Body Composition as a Predictor of Toxicity in Patients Receiving Anthracycline and Taxane-Based Chemotherapy for Early-Stage Breast Cancer. Clin Cancer Res 2017;23:3537-43. [Crossref] [PubMed]
- Stene GB, Helbostad JL, Amundsen T, et al. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol 2015;54:340-8. [Crossref] [PubMed]
- Nattenmüller J, Wochner R, Muley T, et al. Prognostic Impact of CT-Quantified Muscle and Fat Distribution before and after First-Line-Chemotherapy in Lung Cancer Patients. PLoS One 2017;12:e0169136. [Crossref] [PubMed]
- Fabbri E, Chiles Shaffer N, Gonzalez-Freire M, et al. Early body composition, but not body mass, is associated with future accelerated decline in muscle quality. J Cachexia Sarcopenia Muscle 2017;8:490-9. [Crossref] [PubMed]
- Aapro M, Arends J, Bozzetti F, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol 2014;25:1492-9. [Crossref] [PubMed]
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153-66. [Crossref] [PubMed]
- Bruggeman AR, Kamal AH, LeBlanc TW, et al. Cancer Cachexia: Beyond Weight Loss. J Oncol Pract 2016;12:1163-71. [Crossref] [PubMed]
- Vazeille C, Jouinot A, Durand JP, et al. Relation between hypermetabolism, cachexia, and survival in cancer patients: a prospective study in 390 cancer patients before initiation of anticancer therapy. Am J Clin Nutr 2017;105:1139-47. [Crossref] [PubMed]
- Prado CM, Sawyer MB, Ghosh S, et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 2013;98:1012-9. [Crossref] [PubMed]
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11-48. [Crossref] [PubMed]
- Grande AJ, Silva V, Maddocks M. Exercise for cancer cachexia in adults: Executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle 2015;6:208-11. [Crossref] [PubMed]
- Solheim TS, Laird BJA, Balstad TR, et al. Cancer cachexia: rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support Palliat Care 2018;8:258-65. [Crossref] [PubMed]
- Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer 1996;32A:1135-41. [Crossref] [PubMed]
- Chen L, Nelson DR, Zhao Y, et al. Relationship between muscle mass and muscle strength, and the impact of comorbidities: a population-based, cross-sectional study of older adults in the United States. BMC Geriatr 2013;13:74. [Crossref] [PubMed]
- Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006;55:1813-8. [Crossref] [PubMed]
- Norman K, Stobäus N, Reiß J, et al. Effect of sexual dimorphism on muscle strength in cachexia. J Cachexia Sarcopenia Muscle 2012;3:111-6. [Crossref] [PubMed]
- Blauwhoff-Buskermolen S, Langius JAE, Becker A, et al. The influence of different muscle mass measurements on the diagnosis of cancer cachexia. J Cachexia Sarcopenia Muscle 2017;8:615-22. [Crossref] [PubMed]
- Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 2016;17:519-31. [Crossref] [PubMed]
- Crawford J, Prado CM, Johnston MA, et al. Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials). Curr Oncol Rep 2016;18:37. [Crossref] [PubMed]
- Crawford JC, Johnston MA, Hancock ML, et al. Enobosarm, a Selective Androgen Receptor Modulator (SARM) Increases Lean Body Mass (LBM) in Advanced NSCLC Patients; Updated Results of Two Pivotal, International Phase 3 Trials. Presented at the MASCC/ISOO International Symposium on Supportive Care in Cancer. Miami, FL, 2014. Abstract MASCC-0546.
- Crawford J. Cancer cachexia: Are we ready to take a step forward? Cancer 2018;124:456-8. [Crossref] [PubMed]
- Crawford J. Clinical results in cachexia therapeutics. Curr Opin Clin Nutr Metab Care 2016;19:199-204. [PubMed]


