
Global palliative radiotherapy: a framework to improve access in resource-constrained settings
Introduction
Cancer is a global problem, but there is enormous geographic variation in the resources to address it. In 2018, an estimated 43% of new cancer cases and 54% of new cancer deaths occurred in less-developed countries (1). In under-resourced health systems without widely available screening programs, patients with certain cancers, such as breast, cervical, and colorectal cancers, are more likely to present at a late stage and often cannot be treated with curative intent (2,3). This makes comprehensive, palliative approaches that include radiotherapy essential and the dearth of access particularly detrimental.
In all health systems, radiotherapy is a critical part of both curative treatment and palliation of pain and other symptoms for recurrent or metastatic disease (4,5). An estimated 50% of patients will require access to radiotherapy at some time during their treatment course, and this may be as high as 60–70% in low-income countries (6), where patients tend to present at a late stage (5,7).
In 2015, the Union for International Cancer Control’s Global Taskforce on Radiotherapy for Cancer Control (UICC GTFRCC) presented a comprehensive coverage and cost analysis of radiotherapy resources across the globe and a call to action for systematic scale-up (6). However, widely accepted global strategies and targets for radiotherapy access have not yet been developed and adopted. At the country level, among 158 countries with national cancer control or non-communicable disease plans, approximately 20% mentioned radiotherapy, and only 7% detailed how plans would be implemented and monitored (8). Further, while the Lancet Commission on Palliative Care and Pain Relief recently released its findings on access to pain relief and a basic package of palliative interventions, radiotherapy access was not included in the essential package of palliative care (9).
In our previous review, we described how powerful the integration of palliative medicine and palliative radiotherapy could be to improve care for cancer patients globally (5). We included the size of the need for palliative radiotherapy, an analysis of potential barriers and facilitators, and case studies on the emergence of integrated models. In this current review, we will briefly update the current status of access to radiotherapy and palliative care globally, including recent initiatives to quantify and expand access to each. We will then link global radiotherapy and global palliative care initiatives to outline possible next steps toward universal access to palliative radiotherapy with attention to potential synergies between palliative medicine, radiotherapy, and other global health initiatives.
Current status of global radiotherapy and palliative care
Radiotherapy infrastructure and recent developments
Access to palliative radiotherapy depends first on access to basic radiotherapy programs and equipment. The number of high-energy radiotherapy machines per million population has been used to monitor global access by the International Atomic Energy Agency (IAEA)—an international, United Nations-affiliated organization that aims to promote the peaceful use of nuclear technology, including radiotherapy (10-12). The most recent estimates show a significant disparity between high- and low-income settings, with 11.4 machines per million in North America versus 0.05 machines per million in Central Africa (13). Further, there are no machines in nearly forty countries, most of which are low-income. In those countries, national public sector access to referral abroad for treatment is often cost-prohibitive (14).
The GTFRCC estimated radiotherapy coverage per country by accounting for the number of machines per country and making assumptions on operational hours (6). Estimating a 12-hour machine workday in countries with any radiotherapy machines, coverage varied widely from less than 20% in countries with the lowest coverage to nearly 200% in countries with the highest coverage. These coverage estimates, however, may decline when there is a lack of conformity to international guidelines. For example, in a palliative radiotherapy survey of 15 African departments, long palliative radiotherapy schedules for uncomplicated bone metastases were frequently reported rather than the recommended single fraction, thereby decreasing machine availability (15). The authors hypothesize that shorter fractionation schedules are at times not used because of concerns about the possible need for retreatment for patients traveling long distances and because a second RT course is a strain on limited department resources. Further study is needed to define optimum palliative RT practices and indications at radiotherapy centers in low resource settings, findings of which could inform potential strategies for improving resource availability.
National governments and private business initiatives fund most radiotherapy services in the majority low- and middle-income countries (LMICs) (13) and these services also are supported by a number of international organizations. The IAEA supports country-driven initiatives to improve radiotherapy capacity (16) by offering comprehensive assessments of national cancer programs, technical assistance on facility design and financing, staff training and, in unique circumstances, support for machine purchasing. They have also supported numerous efforts to improve capacity, including provider education via virtual case conferences and country-specific assistance on advancing from one modality to another (e.g., two-dimensional radiotherapy to three-dimensional radiotherapy) (17). The IAEA further supports radiotherapy and general radioactive source safety across medical and non-medical applications.
Non-governmental and academic partners have also expanded radiotherapy initiatives over the past decade. There are numerous examples, including the International Cancer Expert Corps (ICEC), Radiating Hope, and the European Society for Radiotherapy and Oncology (ESRTO) Global Impact of Radiotherapy in Oncology (GIRO) initiative. The ICEC was founded in 2013 and aims to engage collaborators from high and low resource settings using a mentorship model to foster expertise and develop technology needed to improve radiotherapy and cancer care (18,19). Radiating Hope was founded in 2007 and aims to raise funds to purchase and donate machines and support equipment maintenance (20). Since its founding, it has expanded into radiotherapy implementation support. Finally, ESTRO’s GIRO was founded in 2015 to utilize existing data to improve upon radiotherapy access with the ultimate goal of saving one million lives by 2035 (21). GIRO will partner with other organizations, including the IAEA, to tailor and strengthen the health policy and investment case for radiotherapy for individual countries worldwide (22). Table 1 highlights the initiatives from these and other organizations and the barriers to implementing palliative radiotherapy in LMICs.
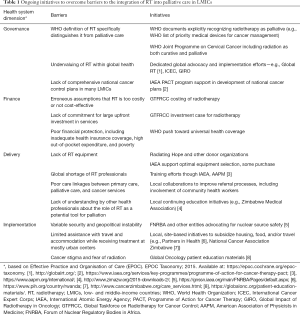
Full table
Despite these initiatives and organizations, there is still no international consensus on how to move forward at the global level. For example, the United Nations Sustainable Development Goals, an agenda for global health and development, include a number of targets for non-communicable diseases and access to healthcare, but they do not explicitly mention radiotherapy or access to cancer care (23). Further, for most LMIC governments, cancer care still remains dauntingly expensive, and any global initiative will require significant funding. There is no large donor fund to provide aid for cancer care similar to the US President’s Emergency Fund for AIDS Relief (PEPFAR) or the Global Fund to Fight AIDS, Tuberculosis, and Malaria (The Global Fund), and many countries do not have universal health insurance to subsidize care (24).
Palliative infrastructure and recent developments
Access to even the most basic palliative care interventions is limited in LMICs. It is estimated that less than one tenth of one percent of all morphine-equivalent medications are distributed in low-income countries (9). National laws and regulations governing opioids typically focus on prevention of diversion and illegal use of opioids and make production importation, and prescription of morphine and other opioids difficult or impossible (25). For this and other reasons, including supply chain logistics and a dearth of manufacturers, stock outs of this key, low cost medication are frequent (9,26). When short-acting formulations are available, patients are frequently given small supplies for fear of diversion (27-31). Long-acting opioid formulations are expensive and even less available in low-income countries (27-31). Often, patients or their family members must travel to central hospitals to refill prescriptions as health centers and regional hospitals are not licensed to dispense or are in short supply (9,27). While access to prescribed opioids for pain due to serious illness had been good in high-resource settings, the recent “opioid crisis” that has emerged in the United States and Canada is increasing fear of opioids and reducing legitimate access for some patients (32,33).
As with radiotherapy, national and local programs have provided most palliative care in low-resource settings. These are largely run by charitable organizations, such as Hospice Africa Uganda, founded in Kampala in 1993 and Pallium India founded in Kerala in 2003 (34,35). Recently, national governments have begun to integrate existing palliative care efforts into the public health system (36-38). Palliative care and hospice organizations in low-resource regional settings have created palliative care networks, including the African Palliative Care Association (APCA) founded in 2002 and the Indian Palliative Care Association (IPCA) founded in 1994 (35,39). These networks enable collaboration and coordination with research and implementation partners and donors from high-resource settings (35,39). They also enable peer support and diffusion of innovation such as more effective models of hospice care or morphine use (39). However, analogous, formal radiotherapy networks have not been established, and palliative care programs often are not integrated with curative therapy (5,40).
In 2017, the Lancet Commission on Palliative Care and Pain Relief published a landmark review of palliative care access in low-resource settings, estimates of palliative care need around the world, and recommendations for making palliative care universally accessible (9). However, although there were commissioners with expertise in radiotherapy and the critical nature of radiotherapy for palliation was discussed in detail, palliative radiotherapy was not included in the recommendations for the basic package. The recommendations focused on a low-cost essential package with small, simple equipment that would require minimal training to use, making it as widely and easily globally applicable as possible. While efforts are underway to simplify radiotherapy delivery through innovation in machine design and automation (19), radiotherapy treatment is still dependent on large, stationary equipment that requires ongoing, high-cost maintenance and specialized expertise to operate.
“Five S” approach: linking analysis to action
The factors that determine access to palliative radiotherapy differ between countries and regions. For example, in many Western countries, palliative radiotherapy as a discipline arose in the context of well-developed radiotherapy systems. The goal was radiotherapy that maximizes symptom relief or prevention, minimizes short-term adverse effects, and minimizes treatment time and burden (41). Integration of palliative care with medical and radiation oncology is increasing (42,43). This integration has resulted in more training on relief of patient and caregiver suffering, on advanced care planning and bereavement care, and on the intricacies of radiotherapy planning (e.g., increased use of stereotactic or more “targeted” approaches) (42).
In LMICs, where radiotherapy often is underdeveloped or unavailable (5,6,15,16), planning for initiation or scale-up of radiotherapy much be integrated from its inception. Development of radiotherapy capacity should be based on an understanding of radiotherapy as an essential and synergistic part of comprehensive cancer care along with medical and surgical oncology and palliative care and on integration of cancer care with non-communicable disease and HIV/AIDS care and with primary care rather than as a separate programmatic silo.
Partners in Health, a non-governmental organization with a long expertise in building health systems in low-resource settings, developed a useful framework for implementing or expanding systems of care such as surgery, mental health, obstetrics, or primary healthcare (44-46).The “Four-S” framework includes staffing, “stuff,” (the equipment and consumables), space (buildings and physical infrastructure), and systems (referral network and logistics). To improve access to global palliative radiotherapy, we propose to add synergies to create a “Five-S” framework (Table 2).

Full table
Staffing
Currently, specialist training in oncology or palliative care is rarely available in LMICs (47,48). However, increasing access to palliative radiotherapy depends on access to training. Regional centers of excellence in oncology could be established at sites already providing high-quality cancer care to train physician-experts in integrated oncology (medical and radiation oncology and palliative care) for a group of LMICs (38). Such centers also could offer basic and intermediate level training for physicians in palliative care, training in oncology and palliative care for nurses, that includes basic information about the palliative benefits of radiotherapy. Physicians and nurses with an understanding both of radiotherapy and of palliative care may help to increase appropriate access to both. In addition, these centers could provide training in medical physics and training for radiation therapists and for biomedical technicians who maintain radiotherapy machines. Often, radiotherapy programs in LMICs rely on a technician employed by the equipment manufacturer who might be based in a distant country (49). Ensuring training and availability of local or national technical support would prevent minor malfunctions from causing long pauses in treatment that result in significant hardship and suffering for patients (50,51).
Within palliative care, there already exist several models for home-based care by health professionals or trained, lay providers. Hospice Africa Uganda, Pallium India, and the national palliative care programs referenced earlier provide a percent of their services to patients at home (36-38). CanSupport, a non-governmental organization in Delhi, provides home-based palliative care with multidisciplinary teams including physicians, nurses, and social workers (52). They provide pain and symptom management, psychosocial support, and bereavement care. From 2009–2010, 746 patients were provided care, with an average of 10 home visits per patient. Further, 71% of patients were self-referred. These existing networks would be amenable to explicit integration of radiotherapy side effects management for those patients referred from radiotherapy departments. They could also potentially be used to identify new patients that might benefit from further evaluation for palliative radiotherapy based on common symptoms (e.g., localized bone pain due to malignancy).
Stuff
In LMICS, reliable and cost-effective palliative care can be provided for most patients with a Cobalt-60 machine or a simple linear accelerator (53). Careful consideration should be given to the security situation in a particular country before choosing a radioactive source modality. Linear accelerators that support artificial intelligence-driven image processing, treatment planning, and quality assurance may enable “tele-radiotherapy”—or the use of telecommunications and information technology to provide radiotherapy support from a distance—and thereby improve both quality and access.
Space
Planning for regional and central cancer centers should include adequate space for radiotherapy.
The cost of establishing radiotherapy programs can be minimized in any setting by adapting for the standardized facility plans developed by IAEA (16). Space also should be designated for patient and family-caregiver housing. Patients and their family caregivers often travel long distances to receive care. Currently, they often sleep outdoors or must pay for admission to the hospital simply so that they may be housed, not for a medical indication. To address this problem at one site, the American Cancer Society (ACS) is raising funds in partnership with Kenyatta National Hospital to build a 62-bed hostel in Nairobi (54).
Systems
Radiotherapy should be thoughtfully and explicitly included in health care system referral patterns and telecommunications. In LMICs with widely distributed populations, telemedicine can enable informed decisions about indications for radiotherapy without requiring patients to make long, costly, and uncomfortable trips for evaluation. In addition, satellite radiotherapy centers capable of managing standard cases and providing palliative care could improve access to radiotherapy at lower cost (55,56).
Beyond telemedicine-based case discussion, other information and communication technologies (ICTs)—defined by Ngwa et al. (57) as technologies that are instrumental in capturing, processing, storage, and exchange of information—also show promise in improving radiotherapy access and quality. Automated contouring and planning could shorten time to treatment and improve standardization, allowing more patients to be treated efficiently. Remote, cloud-based plan review would then allow experts who are not on site to review and revise proposed treatment plans, especially for cases that may require stereotactic or other complex care. Delivered plans and clinical outcomes could then be analyzed using machine learning techniques to more quickly determine the optimal plans and to improve radiotherapy quality and symptom relief over time.
In addition, existing radiotherapy and palliative care centers can be linked in innovative, non-hierarchical ways to promote high-quality cancer care and to address disparities in care services. For example, the National Cancer Grid of India (NCG), founded in 2012, is designed to reduce disparities in quality of care across the country (52). The NCG now includes 143 cancer centers and draws on input from patient advocacy groups, researchers, and other stakeholders to implement evidence-based cancer care guidelines such as those recently published for cervical cancer (53).
Synergies
Synergies are crucial to expansion and improvement of health care systems in LMICs. Synergies that break down traditional disciplinary barriers and integrate care for various conditions can help to optimize use of human resources and reduce costs. Palliative radiotherapy can and should be synergistic and integrated with medical and surgical oncology, palliative care, non-communicable disease, HIV/AIDS, and primary health care.
In LMICs, oncologists are typically trained in both chemotherapy and radiotherapy. In keeping with WHO guidance on integrating palliative care into the responsibilities of any health care provider who cares for people with serious illnesses, oncologists must provide pain and symptom relief (58-60). Especially in settings where there are no palliative care specialists and where patients may pay out of pocket or travel long distances for treatment, such care integration is imperative (15,61).
To promote integrated cancer care in LMICs, integrated cancer care education is needed. Currently, there are many myths and misunderstandings of radiotherapy including that radiation is inherently painful, that it will inevitably cause severe burns, or that it is not compatible with comfort-oriented (6,62,63). Integration of training in radiotherapy, chemotherapy, and palliative care may help to reduce “radiophobia”, improve access to radiotherapy, and promote more rational use.
Efforts to improve access to palliative radiotherapy in LMICs could be strengthened by better integration of radiotherapy with HIV/AIDS care. People with HIV/AIDS often develop cervical cancer, Kaposi sarcoma, anal cancer, or non-Hodgkin’s lymphoma, all of which are amendable to palliative and/or curative radiotherapy. Integration would facilitate care and may help to drive down the cost to the patient of treatment for HIV-related cancers that currently is unsubsidized and prohibitively costly in many LMICs (47). Advocacy for HIV/AIDS treatment that drove down prices and increasing availability of antiretroviral therapy also could improve access to radiotherapy (45,48).
Finally, efforts to achieve universal health coverage (UHC) should include efforts to assure access to radiotherapy (64). The essential package of publicly-funded palliative care recommended by the Lancet Commission on Palliative Care and Pain Relief and by WHO as part of UHC should be augmented as soon as possible to include palliative radiotherapy because of the effectiveness and durability to radio-therapeutic treatment of symptoms such as bone pain and some types of dyspnea and because the same machines that provides palliation also can provide cure in many cases.
Promise and potential barriers
Costs of care
In a few low-income countries, radiotherapy has been funded successfully by the public sector (6,65). In Zambia, for example, close technical and financial collaboration between the government and the IAEA starting in 2002 led to opening the Cancer Diseases Hospital in Lusaka in 2007 with the first in-country radiotherapy services in 2008. Further collaboration is ongoing to develop satellite radiotherapy services to improve access to more of the population. However, because of the cost of scaling up radiotherapy programs, few low-income countries can meet the entirety of their radiotherapy needs with the public health budgets. A “global fund for cancer” has been proposed, modeled on the Global Fund to Fight AIDS, Tuberculosis, and Malaria, which receives four billion US dollars per year and saved an estimated 680,000 million lives from 2003–2007 alone (66-70). Such a fund could enable the lowest income countries to implement radiotherapy for the first time.
Public-private partnerships (PPP), or an agreement between the public sector and a private concern to collaborate on a healthcare endeavor, are another method for financing radiotherapy for low-income patients. This method has been implemented successfully on a small scale in several countries (5,65). Care must be exercised to ensure that these partnerships benefit vulnerable patients in the public sector and not only private enterprise (71,72).
Finally, funding would often be feasible within ministry of health budgets in some cases. Potential barriers, apart from competing health priorities, include knowledge of the benefits of radiation therapy and political will to invest in a service that may take years to implement (6,73). Zambia is an example of such successful commitment. Close technical and financial collaboration between the government and the IAEA starting in 2002 led to opening the Cancer Diseases Hospital in Lusaka in 2007 with the first in-country radiotherapy services in 2008 (74). Further collaboration is ongoing to develop satellite radiotherapy services to improve access to more of the population.
Measuring progress
To assess progress and inform strategic planning, measures of access to palliative radiotherapy must be developed and integrated into routine data collection. In LMICs, these measures might include the need for palliative radiotherapy, current access, the number and distribution of providers trained in radiotherapy and in palliative care, frequency of evidence-based practice, patient outcomes, and costs to patients and families. A model set of indicators could be adapted from the Lancet Commission on Global Surgery, which has developed a comprehensive but concise set that is now being piloted (75). For example, the surgical indictors on timely access and protection of households from impoverishing expenditure could be used for palliative radiotherapy.
Conclusions
Access to palliative radiotherapy is badly needed in resource-limited settings. Without access to this treatment, millions of patients in LMICs and disenfranchised patients in HICs will continue to experience preventable suffering and diminished survival. Integration of radiotherapy, palliative care, medical and surgical oncology, HIV/AIDS care, can improve access and the quality of care. A global fund for cancer care and control could make such integration possible and promote UHC.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- The Global Cancer Observatory (GCO). 2018 ed. Lyon: International Agency for Research on Cancer (IARC). Available online: http://gco.iarc.fr/
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Jedy-Agba E, McCormack V, Adebamowo C, et al. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2016;4:e923-35. [Crossref] [PubMed]
- Jacob S, Wong K, Delaney GP, et al. Estimation of an Optimal Utilisation Rate for Palliative Radiotherapy in Newly Diagnosed Cancer Patients. Clin Oncol (R Coll Radiol) 2010;22:56-64. [Crossref] [PubMed]
- Rodin D, Grover S, Elmore SN, et al. The power of integration: radiotherapy and global palliative care. Ann Palliat Med 2016;5:209-17. [Crossref] [PubMed]
- Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol 2015;16:1153-86. [Crossref] [PubMed]
- Barton MB, Frommer M, Shafiq J. Role of radiotherapy in cancer control in low-income and middle-income countries. Lancet Oncol 2006;7:584-95. [Crossref] [PubMed]
- Romero Y, Trapani D, Johnson S, et al. National cancer control plans: a global analysis. Lancet Oncol 2018;19:e546-55. [Crossref] [PubMed]
- Knaul FM, Farmer PE, Krakauer EL, et al. Alleviating the access abyss in palliative care and pain relief-an imperative of universal health coverage: the Lancet Commission report. Lancet 2018;391:1391-454. [Crossref] [PubMed]
- Rosenblatt E, Acuña O, Abdel-Wahab M. The challenge of global radiation therapy: an IAEA perspective. Int J Radiat Oncol Biol Phys 2015;91:687-9. [Crossref] [PubMed]
- Abdel-Wahab M, Bourque JM, Pynda Y, et al. Status of radiotherapy resources in Africa: an International Atomic Energy Agency analysis. Lancet Oncol 2013;14:e168-75. [Crossref] [PubMed]
- Rosenblatt E, Izewska J, Anacak Y, et al. Radiotherapy capacity in European countries: an analysis of the Directory of Radiotherapy Centres (DIRAC) database. Lancet Oncol 2013;14:e79-86. [Crossref] [PubMed]
- DIRAC (DIrectory of RAdiotherapy Centres). International Atomic Energy Agency. Available online: http://www-naweb.iaea.org/nahu/dirac/default.asp
- Zubizarreta EH, Fidarova E, Healy B, et al. Need for Radiotherapy in Low and Middle Income Countries - The Silent Crisis Continues. Clin Oncol (R Coll Radiol) 2015;27:107-14. [Crossref] [PubMed]
- Sharma V, Gaye PM, Wahab SA, et al. Patterns of Practice of Palliative Radiotherapy in Africa, Part 1: Bone and Brain Metastases. Int J Radiat Oncol Biol Phys 2008;70:1195-201. [Crossref] [PubMed]
- Enwerem-Bromson N, Abdel-Wahab M. Expanding global access to radiotherapy: the IAEA perspective. Lancet Oncol 2015;16:1151-2. [Crossref] [PubMed]
- Rosenblatt E, Prasad RR, Hopkins K, et al. Africa Radiation Oncology Network (AFRONET): an IAEA Telemedicine Pilot Project. JISfTeH 2018;6:e61-7.
- Coleman CN, Formenti SC, Williams TR, et al. The international cancer expert corps: a unique approach for sustainable cancer care in low and lower-middle income countries. Front Oncol 2014;4:333. [Crossref] [PubMed]
- Pistenmaa DA, Dosanjh M, Amaldi U, et al. Changing the global radiation therapy paradigm. Radiother Oncol 2018;128:393-9. [Crossref] [PubMed]
- Dad L, Shah MM, Mutter R, et al. Why target the globe?: 4-year report (2009-2013) of the Association of Residents in Radiation Oncology Global Health Initiative. Int J Radiat Oncol Biol Phys 2014;89:485-91. [Crossref] [PubMed]
- Lievens Y, Gospodarowicz M, Grover S, et al. Global impact of radiotherapy in oncology: Saving one million lives by 2035. Radiother Oncol 2017;125:175-7. [Crossref] [PubMed]
- Rodin D, Abdel-Wahab M, Lievens Y. Global radiotherapy challenge: turning data into action. Lancet Glob Health 2018;6:S15-6. [Crossref]
- The Sustainable Development Goals Report 2017. Geneva: World Health Organization 2017;1-64.
- Hogan DR, Stevens GA, Hosseinpoor AR, et al. Articles Monitoring universal health coverage within the Sustainable Development Goals: development and baseline data for an index of essential health services. Lancet Glob Health 2018;6:e152-68. [Crossref] [PubMed]
- World Health Organization. Ensuring balance in national policies on controlled substances. Geneva: WHO Press;2011:1-86.
- Cleary JF, Maurer MA. Pain and Policy Studies Group. Two Decades of Working to Address Regulatory Barriers to Improve Opioid Availability and Accessibility Around the World. J Pain Symptom Manage 2018;55:S121-34. [Crossref] [PubMed]
- Cleary J, Powell RA, Munene G, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Africa: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol 2013;24:xi14-23. [Crossref] [PubMed]
- Cleary J, Radbruch L, Torode J, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Asia: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol 2013;24:xi24-32. [Crossref] [PubMed]
- Cleary J, Simha N, Panieri A, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in India: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol 2013;24:xi33-40. [Crossref] [PubMed]
- Cleary J, De Lima L, Eisenchlas J, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Latin America and the Caribbean: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol 2013;24:xi41-50. [Crossref] [PubMed]
- Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol 2013;24:xi51-9. [Crossref] [PubMed]
- Bruera E, Del Fabbro E. Pain Management in the Era of the Opioid Crisis. Am Soc Clin Oncol Educ Book 2018.807-12. [Crossref] [PubMed]
- Gilson AM, Maurer MA, Ryan KM, et al. Using a Morphine Equivalence Metric to Quantify Opioid Consumption: Examining the Capacity to Provide Effective Treatment of Debilitating Pain at the Global, Regional, and Country Levels. J Pain Symptom Manage 2013;45:681-700. [Crossref] [PubMed]
- Mwangi-Powell FN, Powell RA, Harding R. Models of delivering palliative and end-of-life care in sub-Saharan Africa: a narrative review of the evidence. Curr Opin Support Palliat Care 2013;7:223-8. [Crossref] [PubMed]
- Rajagopal M. The current status of palliative care in India. Cancer Management 2015;57-61.
- Krakauer EL, Muhimpundu MA, Mukasahaha D, et al. Palliative Care in Rwanda: Aiming for Universal Access. J Pain Symptom Manage 2018;55:S77-80. [Crossref] [PubMed]
- Luyirika EB, Namisango E, Garanganga E, et al. Best practices in developing a national palliative care policy in resource limited settings: lessons from five African countries. Ecancermedicalscience 2016;10:652. [Crossref] [PubMed]
- Krakauer EL, Thinh DH, Khanh QT, et al. Palliative Care in Vietnam: Long-Term Partnerships Yield Increasing Access. J Pain Symptom Manage 2018;55:S92-5. [Crossref] [PubMed]
- Mwangi-Powell F. Palliative Care and Public Health, A Perspective from the African Palliative Care Association. J Public Health Policy 2007;28:59-61. [Crossref] [PubMed]
- Krakauer EL. Just palliative care: responding responsibly to the suffering of the poor. J Pain Symptom Manage 2008;36:505-12. [Crossref] [PubMed]
- Lutz S, Chow E. Palliative radiotherapy: past, present and future-where do we go from here? Ann Palliat Med 2014;3:286-90. [PubMed]
- Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: Report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol 2017;7:113-9. [Crossref] [PubMed]
- Lutz ST, Jones J, Chow E. Role of Radiation Therapy in Palliative Care of the Patient With Cancer. J Clin Oncol 2014;32:2913-9. [Crossref] [PubMed]
- Boozary AS, Farmer PE, Jha AK. The Ebola Outbreak, Fragile Health Systems, and Quality as a Cure. JAMA 2014;312:1859-60. [Crossref] [PubMed]
- Elmore SN, Sethi RV, Viswanathan AN, et al. Global Radiation Oncology From the Trainee Perspective: A View From Beyond the Bunker. Int J Radiat Oncol Biol Phys 2016;94:438-9. [Crossref] [PubMed]
- Bitton A, Veillard JH, Basu L, et al. The 5S-5M-5C schematic: transforming primary care inputs to outcomes in low-income and middle-income countries. BMJ Glob Health 2018;3:e001020-4. [Crossref] [PubMed]
- Adebamowo CA, Casper C, Bhatia K, et al. Challenges in the detection, prevention, and treatment of HIV-associated malignancies in low- and middle-income countries in Africa. J Acquir Immune Defic Syndr 2014;67 Suppl 1:S17-26. [Crossref] [PubMed]
- Rabkin M, El-Sadr WM. Why reinvent the wheel? Leveraging the lessons of HIV scale-up to confront non-communicable diseases. Glob Public Health 2011;6:247-56. [Crossref] [PubMed]
- Elmore SN, Sethi RV, Kavuma A, et al. Broken Machines or Broken Systems: The Road to Meaningful Global Radiotherapy Access. J Glob Oncol 2016;3:438-40. [Crossref] [PubMed]
- Rosenblatt E. Planning national radiotherapy services. Front Oncol 2014;4:315. [Crossref] [PubMed]
- Eder-Van Hook J, Love C. A Model for Training Biomedical Equipment Technicians in Low-Resource Settings. San Diego: Transition Management Consulting Inc., 2015;1-95.
- Yeager A, LaVigne AW, Rajvanshi A, et al. CanSupport: a model for home-based palliative care delivery in India. Ann Palliat Med 2016;5:166-71. [Crossref] [PubMed]
- Page BR, Hudson AD, Brown DW, et al. Cobalt, linac, or other: what is the best solution for radiation therapy in developing countries?. Int J Radiat Oncol Biol Phys 2014;89:476-80. [Crossref] [PubMed]
- American Cancer Society. KNH Hope Hostel. Available online: https://www.cancer.org/health-care-professionals/our-global-health-work/kenyatta-national-hospital-hope-hostel.html
- Datta NR, Heuser M, Bodis S. A Roadmap and Cost Implications of Establishing Comprehensive Cancer Care Using a Teleradiotherapy Network in a Group of Sub-Saharan African Countries With No Access to Radiation Therapy. Int J Radiat Oncol Biol Phys 2016;95:1334-43. [Crossref] [PubMed]
- Datta NR, Heuser M, Samiei M, et al. Teleradiotherapy Network: Applications and Feasibility for Providing Cost-Effective Comprehensive Radiotherapy Care in Low- and Middle-Income Group Countries for Cancer Patients. Telemedicine and e-Healt 2015;21:523-32.
- Ngwa W, Sajo E, Ngoma T, et al. Potential for information and communication technologies to catalyze global collaborations in radiation oncology. Int J Radiat Oncol Biol Phys 2015;91:444-7. [Crossref] [PubMed]
- Mathew A. Global Survey of Clinical Oncology Workforce. J Glob Oncol 2018.1-12. [Crossref] [PubMed]
- Osman H, Shrestha S, Temin S, et al. Palliative Care in the Global Setting: ASCO Resource-Stratified Practice Guideline. J Glob Oncol 2018.1-24. [PubMed]
- Cleary J, Ddungu H, Distelhorst SR, et al. Supportive and palliative care for metastatic breast cancer: Resource allocations in low- and middle-income countries. A Breast Health Global Initiative 2013 consensus statement. Breast 2013;22:616-27. [Crossref] [PubMed]
- Jeremic B, Vanderpuye V, Abdel-Wahab S, et al. Patterns of Practice in Palliative Radiotherapy in Africa - Case Revisited. Clin Oncol (R Coll Radiol) 2014;26:333-43. [Crossref] [PubMed]
- Dharmarajan KV, Rich SE, Johnstone CA, et al. Top 10 Tips Palliative Care Clinicians Should Know About Radiation Oncology. J Palliat Med 2018;21:383-8. [Crossref] [PubMed]
- Rayne S, Schnippel K, Firnhaber C, et al. Fear of Treatments Surpasses Demographic and Socioeconomic Factors in Affecting Patients With Breast Cancer in Urban South Africa. J Glob Oncol 2016;3:125-34. [Crossref] [PubMed]
- World Health Organization. Draft thirteenth general programme of work 2019-2023. 2018. Available online: http://apps.who.int/gb/ebwha/pdf_files/EB142/B142_3-en.pdf
- Kaliks RA, Pontes L de B, Bognar CL, et al. Treatment of breast cancer patients from a public healthcare system in a private center: costs of care for a pilot public-private partnership in oncology. Einstein (São Paulo). 9 ed. Einstein (Sao Paulo) 2013;11:216-23. [Crossref] [PubMed]
- Cavalli F, Atun R. Towards a global cancer fund. Lancet Oncol 2015;16:133-4. [Crossref] [PubMed]
- Results Report 2017. The Global Fund 2017. Available online: https://www.aidsdatahub.org/sites/default/files/publication/Global_Fund_Results_report_2017.pdf
- Winters J, Sridhar D. Earmarking for global health: benefits and perils of the World Bank's trust fund model. BMJ 2017;358:j3394. [Crossref] [PubMed]
- Sachs JD, Schmidt-Traub G. Global Fund lessons for Sustainable Development Goals. Science 2017;356:32-3. [Crossref] [PubMed]
- Komatsu R, Korenromp EL, Low-Beer D, et al. Lives saved by Global Fund-supported HIV/AIDS, tuberculosis and malaria programs: estimation approach and results between 2003 and end-2007. BMC Infect Dis 2010;10:109. [Crossref] [PubMed]
- Webster PC. World Report Lesotho’s controversial public-private partnership project. Lancet 2015;386:1929-31. [Crossref] [PubMed]
- Allen L, Bloomfield A. Engaging the private sector to strengthen NCD prevention and control Comment. Lancet Glob Health 2016;4:e897-8. [Crossref] [PubMed]
- Ferraz MB, Azevedo RT. Ministers of Health: short-term tenure for long-term goals? Sao Paulo Med J 2011;129:77-84. [Crossref] [PubMed]
- Abdel-Wahab M, Zubizarreta E, Polo A, et al. Improving Quality and Access to Radiation Therapy—An IAEA Perspective. Semin Radiat Oncol 2017;27:109-17. [Crossref] [PubMed]
- Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 2015;386:569-624. [Crossref] [PubMed]


