Changes in expression of cyclooxygenase-2 in the spinal dorsal horn after intrathecal p38MAPK inhibitor SB203580 on neuropathic pain in rats
In the complex mechanism of the development of neuropathic pain, p38MAPK is an important signaling pathway (1). The activation of p38MAPK plays an essential role in the generation and maintenance of neuropathic pain by regulating cellular transcription, protein synthesis, and receptor expression (2-4). Cyclooxygenase-2 (COX-2) is one of the important rate-limiting enzymes in the synthesis of prostaglandin (PG), a pain inducing substance, and is also the target of many non-steroidal analgesics. A study (5) has showed significantly increased COX-2 positive cells on the first day and from day 7 to 14 following nerve injury, which is associated the formation of neuropathic pain. However, there are few reports as to whether p38MAPK acts by regulating the expression of COX-2 proteins. This study is designed to identify the impact of intrathecal SB203580 on COX-2 expression in the spinal dorsal horn of rats with chronic constriction injury, and to determine whether the expression of COX-2 proteins is regulated by p38MAPK.
Materials and methods
Animals
Clean grade male SD rats, weighing 200 to 220 g, were purchased from Dongchuang Lab Animal Ltd., Changsha, Hunan.
Materials
SB203580 (Promega, USA) was dissolved in 2% dimethyl sulfoxide. COX-2 antibodies were purchased from Santa Cruz, USA; the DAB kit was purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. The plantar test instrument was purchased from IITC, USA.
Methods
The intrathecal catheter was placed using the approach suggested by Fan Long, et al. (6). After anesthesia with intraperitoneal injection of 300 mg/kg 10% chloral hydrate, the rats were fixed in the prone position with a 20 mL under the abdomen to raise the waist. Skin preparation and sterilization was performed with the L4 and L5 spinous space as the center. The spinal needle stylet was inserted into a self-made intrathecal catheter. Two sutures were attached at 3.5 cm of the catheter in advance. A 1 cm longitudinal incision was made to the skin over the L4 and L5 spinous space. Part of the subcutaneous fascia and supraspinal ligament was cut to expose the interspinous ligament. A size 5 steel needle was probed into the interspinous ligament until slight shaking of the rat tail was observed. The intrathecal catheter with a spinal needle core inside was inserted along the needle probe until the above dural irritation was present. While gently pushing the catheter with the left hand, the spinal needle core was extracted with the right hand. The catheter was inserted so deep that the sutures were buried between the muscles and ligaments. The sutures previously attached in the catheter were introduced from the muscular layer through the deep fascia from both sides with a round needle, and knotted for fixation of the catheter. The intrathecal catheter was inserted into the outer sleeve and fixed with 502 super glue. The epidural catheter sleeve was inserted from the neck through the subcutaneous fascia to the waist incision. With the catheter placed inside, the sleeve was retracted through the previous route to introduce the catheter through to the neck. Local suture was applied for fixation. The incision was closed layer by layer. The catheter was washed with 10 µL saline using a micro-injector, and sealed with a steel pipe plug. Intramuscular injection of 80,000 U penicillin sodium was administered for each rat after surgery, twice every day for three consecutive days. All rats were kept separately in individual cages. After the rats were fully awake, intrathecal injection of 20 µL 2% lidocaine was given. Successful catheterization was achieved in those presenting lower limb paralyses in 7 to 20 s and relieved in 30 min. After three days, rats that had lost limb sensory or motor functions, and still had the catheters in place, were selected into the experiment.
Twnety-four male SD rats with successful intrathecal catheterization were randomly divided into four groups (n=6): the sham operation group (sham group), normal saline group (NS group), 2% dimethyl sulfoxide group (DMSO group), and SB203580 group (SB group). Rats in the NS group, DMSO group and SB group were prepared using the method by Bennett et al. (7) to establish the chronic constriction injury (CCI) models. After anesthesia using intraperitoneal injection of 300 mg/kg 10% chloral hydrate, a rat was placed in left lateral decubitus. The skin on the right thigh was prepared and sterilized. A longitudinal incision of approximately 1.5 cm was made in the middle of the right thigh. The fascia was separated, and the muscles were divided posterior to the femoral bone to expose the right sciatic nerve. The sciatic nerve was separated close to but before the bifurcation. A 4-0 silk suture was used to loosely ligate for sites at an interval of 1 mm under microscope to cause slight convulsions in the leg without affecting the blood supply to the epineurium. The area was then covered with penicillin powder and sutured. The mechanical withdrawal threshold (MWT) was measured before and after CCI. A CCI model was considered successful when a decline of 40% was observed in the MWT (8). For the sham group, the sciatic nerve was exposed without being ligated.
MWT measurements were performed on both the operated and contralateral sides in all rats one day before and on days 1, 3, 5, 7, 9 and 11 after the CCI surgery. The preoperative measurement was the baseline threshold value. MWT values were measured using the electro-mechanical Plantar test device (9). A rat was placed in a glass box with a metal bottom, and direct stimulation was provided to the plantar area via a measuring probe to record the maximum contraction threshold as the paw contracted. Both lateral plantar parts were subject to the test at an interval of 3 minutes for three times. The values were averaged as the final result.
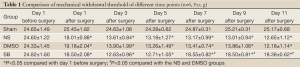
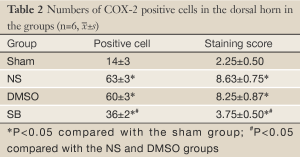
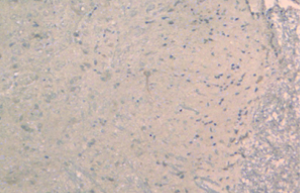
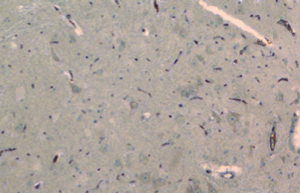
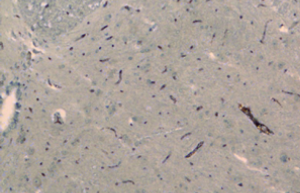
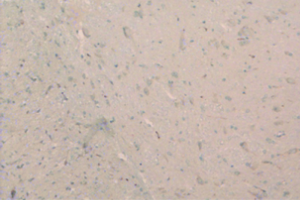
From day 6 after surgery, NS 10 µL was administered to the NS group, 10 µL 2% dimethyl sulfoxide to the DMSO group, and SB203580 5 µg/10 µL to the SB group. An additional 10 µL NS was injected following the above doses to seal the catheter. These injections were delivered twice every day for five consecutive days. No drug was injected for the sham group. After behavioral observation on day 11 postoperatively, the rats were sacrificed and the spinal cord specimens were taken for immunological examination. The procedure was as follows: thoracotomy was performed under deep anesthesia with intraperitoneal injection of 350 mg/kg 10% chloral hydrate, followed by catheterization via the left ventricle to the ascending aorta. After blood was washed using natural saline, the tissue was perfusion-fixed with 200 mL 4% paraformaldehyde in 0.1% mol/L PBS buffer (pH =7.4). The spinal cord at the lumbar enlargement was fixed in the above fixative for 24 hours. The tissue was subject to graded alcohol dehydration, xylene cleaning, paraffin embedding, and made into continuous coronal slices with a thickness of 5 µm. Expression levels of COX-2 proteins at the spinal dorsal horn of the operative side were measured. Immunohistochemical analysis was performed using the method described in (10). Four fields were randomly selected from the operated side on the shallow layer of the spinal dorsal horn under low magnification (×100). The number of COX-2 protein immunoreactive (positive) cells, as defined by presence of brown particles in the cytoplasm, per 100 cells was counted using the double-blind method. Immunohistochemical staining scores were calculated based on the percentage and staining intensity of positive cells: (I) percentage of positive cells (0 for no positive cells; 1 for 1-10%; 2 for 11-50%; 3 for 51-80%; 4 for 81-100%); (II) staining intensity [0 for negative; 1 for weakly positive (light yellow); 2 for moderately positive (yellow); 3 for strongly positive (brown)]; the product of (I) and (II) was the immunohistochemical staining score for the specific slice. In the case of differing scores for a single field, the average value was used.
Statistical analysis
Statistical analysis was performed using SPSS13.0. All measurement data were expressed as mean ± standard deviation (). Pairwise comparison between groups was conducted using analysis of variance, with a P of less than 0.05 being considered statistically significant.
Results
All rats survived well after surgery without wound infection. No significant decrease in body weight was observed. Except for the sham group, the rats in the other three groups appeared to be reluctant to touch the ground with their right hind paws, and the affected legs were contracted. The symptoms improved in SB group after the injection.
Compared with the sham group, lower MWT measurements were observed at all time points in the NS, DMSO and SB groups (P<0.05). Compared with the NS and DMSO groups, higher MWT values were observed in the SB group after intrathecal injection (P<0.05; Table 1).
There was no significant difference in the COX-2 positive cell count and staining score between the NS group and DMSO group (P>0.05). Compared with these two groups, significantly lower positive cells were found in the SB group, as well as the staining store (P<0.05; Table 2, Figures 1-4).
Discussion
CCI is a classical model in the study of neuropathic pain. This study is based on the method suggested by Bennett et al. (7). To ensure consistent tightness in the experiment, the ligation was performed from proximal to distal ends under microscope to the extent that slight convulsions were observed in the specific limb without compromising the blood supply to the epineurium. After surgery, the rats appeared to be reluctant to touch the ground with their right hind paws, and the affected legs were contracted, in opposite to the contralateral side. That was consistent with the previous report of CCI models (7). Except for the sham group, MWT measurements decreased by more than 40% in the affected legs of the rats in the other groups, indicating that the CCI model developed in this study was successful.
In the process of development of neuropathic pain, p38MAPK plays an important role. Following nerve injury, p38MAPK is activated via multiple pathways, and p38MAPK in turn contributes to the maintenance phase of neuropathic pain through activation of the transcription factor NF-κB to regulate nuclear gene transcription (11,12) and protein translation. It can be seen that p38MAPK is involved in the regulation of nuclear transcription factor cAMP response element binding protein (CREB), nitric oxide synthase (iNOS) (13) and Na (v) 1.8 sodium channels. Zhang Feie and colleagues found in a study (8) that intrathecal injection of a p38MAPK inhibitor, SB203580, could reduce CCI-induced hyperalgesia, and inhibits the increased expression of pCREB in the spinal dorsal horn in CCI model rats. They suggested that CREB could be one of the important downstream factors of p38MAPK. Hudmon et al. (14) found that in the dorsal root ganglia, a common area of the p38MAPK and Na (v) 1.8 sodium channels, the Na (v) 1.8 sodium channels in the dorsal root ganglion served as a p38MAPK substrate, and their functions could be adjusted through phosphorylation.
In this study, the COX-2 protein expression was increased in the spinal dorsal horn of rats after CCI surgery, suggesting that the COX-2 in the spinal cord had an important role in neuropathic pain. A previous study (15) reported that in the CCI pain model, the COX-2 protein expression in the microglial cells of the spinal cord dorsal horn was significantly increased on days 4, 21 and 30 after ligation, which supported the results of this study. SB203580 is a specific inhibitor of p38MAPK. By inhibiting p38MAPK phosphorylation, it reduces the levels of phosphorized p38MAPK and blocks the p38MAPK downstream signaling cascades. Based on the results in this study, compared with the NS group and DMSO group, the SB group was associated with reduced expression of COX-2 proteins in the spinal cord dorsal horn after intrathecal injection of SB203580, suggesting that the noxious stimulation following CCI surgery led to p38MAPK phosphorylation of the spinal cord dorsal horn and activation of the p38MAPK signal transduction pathway, which in turn triggered neuropathic pain through regulation of COX-2 protein expressions and many other pathways. By inhibiting the activation of p38MAPK, the COX-2 protein expression level was reduced, and neuropathic pain was thereby effectively alleviated.
In summary, intrathecal injection of SB203580 can reduce COX-2 protein expression in CCI rats, suggesting that p38MAPK is involved in the regulation of COX-2 protein expression.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain 2007;3:33. [PubMed]
- Suzuki T, Hide I, Ido K, et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci 2004;24:1-7. [PubMed]
- Wu J, Xu Y, Pu S, et al. p38/MAPK inhibitor modulates the expression of dorsal horn GABA(B) receptors in the spinal nerve ligation model of neuropathic pain. Neuroimmunomodulation 2011;18:150-5. [PubMed]
- Lee KM, Jeon SM, Cho HJ. Interleukin-6 induces microglial CX3CR1 expression in the spinal cord after peripheral nerve injury through the activation of p38 MAPK. Eur J Pain 2010;14:682.e1-12.
- Takahashi M, Kawaguchi M, Shimada K, et al. Cyclooxygenase-2 expression in Schwann cells and macrophages in the sciatic nerve after single spinal nerve injury in rats. Neurosci Lett 2004;363:203-6. [PubMed]
- Fan L, Wang G. Development of catheterization for subarachnoid space in rats. Journal of Tianjin Medical University 2005;11:165-7.
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988;33:87-107. [PubMed]
- Zhang FE, Zhang LC, Jia JT. Analgesic effect of intrathecal SB203580 in a rat model of neuropathic pain. Chin J Anesthesiol 2006;26:356-9.
- Vivancos GG, Verri WA Jr, Cunha TM, et al. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res 2004;37:391-9. [PubMed]
- Soslow RA, Dannenberg AJ, Rush D, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 2000;89:2637-45. [PubMed]
- Jana M, Dasgupta S, Saha RN, et al. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem 2003;86:519-28. [PubMed]
- Wilms H, Rosenstiel P, Sievers J, et al. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease. FASEB J 2003;17:500-2. [PubMed]
- Sung CS, Wen ZH, Chang WK, et al. Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. J Neurochem 2005;94:742-52. [PubMed]
- Hudmon A, Choi JS, Tyrrell L, et al. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci 2008;28:3190-201. [PubMed]
- Durrenberger PF, Facer P, Casula MA, et al. Prostanoid receptor EP1 and Cox-2 in injured human nerves and a rat model of nerve injury: a time-course study. BMC Neurol 2006;6:1. [PubMed]