The use of opioids at the end-of-life and the survival of Egyptian palliative care patients with advanced cancer
Introduction
Cancer pain can be controlled in the majority of patients using simple drugs and following readily available guidelines (1). However, cancer pain control remains inadequate worldwide due to many barriers including lack of education about the use of opioids for cancer pain management which resulted in misconceptions and unfounded fears (2). One of these misconceptions is that opioids may hasten the death of patients with advanced cancer, especially when used at higher doses (3). Some studies were conducted in higher-income countries to refute the misconception that opioids used to manage cancer pain in patients with advanced cancer would hasten death (4-14).
Cancer pain control in Egypt is largely inadequate as indicated by the very low opioid consumption figures (15). This is due to many barriers, especially those related to opioids availability and accessibility (16-18).
The establishment of a palliative medicine unit (PMU) in an Egyptian cancer center was associated with a significant increase in opioid consumption where the consumption increased by sevenfold (19). In another study from the same center, the opioid dose was increased to ≥300 mg oral morphine equivalent (OME)/24 h in 19% of palliative care (PC) patients with advanced cancer (18). The increase in opioid consumption after the establishment of the PMU and the administration of relatively higher doses of opioids to a significant proportion of patients raised concerns about the impact of opioids on the survival of patients with advanced cancer in our setting.
The aim of this study was to explore the relationship between the opioid dose and the survival of Egyptian patients with advanced cancer managed in a PMU.
Methods
A retrospective study that included patients with advanced cancer referred to a cancer center-based PMU in Egypt. To be eligible for the study, patients should be adults (age ≥18 years), have a confirmed advanced incurable cancer diagnosis and confirmed death.
During the study period the following opioids were available at our center: slow release morphine tablets (30 mg), transdermal fentanyl patches (50 microgram/h) and tramadol of different formulations. The last prescribed regular opioid dose was the one taken into consideration and was expressed in milligrams of OME per day (mg OME/24 h). To calculate the OME, we used conversion ratios of 1:100 to convert from TDF to oral morphine (20), and 10:1 to convert from tramadol to oral morphine (21).
The cut-off points used to group patients according to the last prescribed opioid dose was similar to that used in previous studies (9,11). The three groups were no/low-dose opioid group (<120 mg OME/24 h), intermediate-dose group (120-<300 mg OME/24 h) and high-dose group (≥300 mg OME/24 h).
The survival of patients was calculated from the date of first referral to the PMU to death. A P value <0.05 was considered significant.
Statistical methods were performed using the Statistical Package for the Social Sciences (SPSS), version 14.0 (SPSS Inc, Chicago, Illinois).
The study was approved by the ethics committee of the Kasr Al-Ainy Center of Clinical Oncology and Nuclear Medicine, Kasr Al-Ainy School of Medicine, Cairo University.
Results
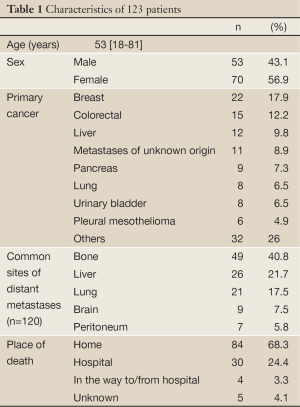
The study included 123 non-consecutive patients with advanced cancer for whom the death event was confirmed in the period from October 2008 to July 2011. The characteristics of patients are shown in Table 1. Excluding three (2.4%) patients with hematological malignancies, 82 (68.3%) of the remaining 120 patients with solid tumors had evidence of distant metastases.
Full Table
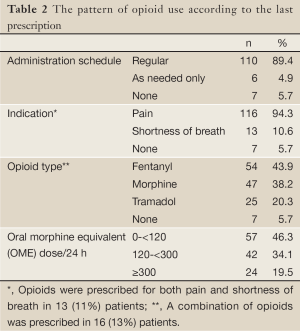
The pattern of opioid use according to the last documented prescription is shown in Table 2. Among patients for whom opioids were prescribed on regular basis, 88 (71%) patients were receiving a strong opioid and 22 (18%) patients were receiving a weak opioid only.
Full Table
For the whole group of patients, the mean last prescribed opioid dose was 167 (±170) mg OME/24 h.
There was no significant correlation between age and the last prescribed opioid dose (P=0.38). Similarly, the mean last prescribed opioid dose did not differ significantly according to sex (P=0.26) or place of death (home vs. others) (P=0.92). The mean last prescribed dose was highest for patients with pleural mesothelioma [245 (±258) mg OME/24 h] followed by those with pancreatic cancer [243 (±179) mg OME/24 h] and was lowest for patients with liver cancer [80 (±84) mg OME/24 h]. However, the difference in the opioid dose according to the site of primary cancer was not statistically significant (P=0.54).
The opioid dose was significantly higher among patients with bone metastases compared to those without [206 (±178) vs. 142 (±161) mg OME/24 h, respectively; P=0.041] and was significantly lower in those with liver metastases compared to those without [103 (±101) vs. 184 (±181) mg OME/24 h, respectively; P=0.03]. The opioid dose did not differ significantly according to the presence of lung, brain or peritoneal metastases (P=0.97, 0.21 and 0.21, respectively).
The overall estimated survival from the date of first referral to the PMU until death was 66 days (95% CI: 53-79 days).
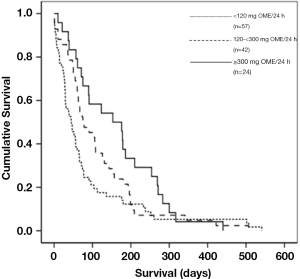
The Kaplan Meier survival curves for the three groups of patients are shown in Figure 1.
The estimated median survival was highest for the high-dose group [153 days (95% CI: 49-257 days)] followed by the intermediate-dose group [75 days (95% CI: 33-117 days)] and the no/low-dose group [45 days (95% CI: 23-67 days)]. The difference between the three groups was statistically significant (P=0.031).
Discussion
To the best of our knowledge, the current study is the first from a lower-income country to investigate the relation between the survival of patients with advanced cancer and the dose of opioids administered at the end-of-life in a PC setting.
The other reported studies were conducted in higher-income countries where PC is relatively well established (4-14). The conduction of a similar study in countries where PC is in an early stage of development, like Egypt, is important to overcome opiophobia which represents a major barrier to PC development and cancer pain control (2).
The results of the current study confirm the findings in other studies that the survival of patients receiving higher doses of opioids is not shorter than that of those receiving lower doses or did not receive opioids (5,7-9,12,13).
Researchers used different approaches in different settings to investigate the impact of using opioids on the survival of patients dying of cancer.
The time point from which survival was calculated was relatively late in the trajectory of the disease in other studies (7,9,12). For example, Morita et al. reported a median survival of 26 days calculated from the date of admission to a PC unit to death (7). Similarly, Good et al. calculated the survival from the date of hospice admission with a median of 8 days (9). On the other hand, Bengoechea et al. reported the median survival from the day of maximum opioid dose to death and was 2 and 6 days for the two groups of patients described (12). This may explain, in part, the longer median survival (66 days) reported in our study as we calculated the survival from the date of first referral to the PMU which occurs in the outpatient clinic in the majority of our patients (22). Another reason for the relatively longer survival in our study may be the earlier referral to the PMU in our setting. This is supported by the findings that the median survival was only 5 days when it was calculated by Radha Krishna et al. from the time of PC referral, a point similar to ours (13). Our results provide new information on the relation between opioids and survival by studying it in a PC cancer population with relatively longer PC survival.
Most of previous studies included exclusively inpatients who died in specialized PC facilities (5-7,9,11,14). Only two studies included patients managed by PC teams at home (8,12). Because the majority of our PC patients die at home (22), it was important to include home deaths in our study which represented 68% of all patients. Of note, there was no significant difference in the mean last prescribed opioid dose according to the place of death in our study. The current results add to the evidence suggesting that using opioids at the end-of-life has no detrimental effect on survival among cancer patients who die at home.
Researchers used two main approaches to assess the impact of opioids on the survival of terminally-ill cancer patients. The first approach was to study the relation between survival and the “opioid dose” administered at the end-of-life (5,7-9). The other approach was to study the relation between survival and the “change in opioid dose”, rather than the dose itself, at the end-of-life (6,11,12). It had been suggested that using the “opioid dose” for comparison may be misleading and that using the “change in opioid dose” is more accurate (3). However, in the current study we used the “opioid dose” rather than the “change in opioid dose” for comparison. The reason behind that is with the start of our PMU there was much concern towards using high dose opioids for cancer pain control which was a new practice in our setting (16), and we found it a priority to address that concern.
Some of the studies that assessed survival according to the opioid dose found no significant correlation between opioid dose and survival (5,7,12). Other studies found, unexpectedly, that higher opioid doses are associated with significantly longer survival (8,9,13). In the study conducted by Bercovitch et al., the median survival of patients who received high doses (300-599 mg/day) and very high doses (≥600 mg/day) of morphine was significantly longer than that of patients who received lower doses or did not receive morphine (8). Similarly, Good et al. found that patients treated with ≥300 mg OME/day had a significantly longer survival compared to those treated with 120-299 mg OME/day and <120 mg OME/day (9). Our results were concord with these results. We found that high opioid dose (≥300 mg OME/day) was associated with longer survival compared to intermediate (120-300 mg OME/day) and low opioid dose/no opioids (<120 mg OME/day) and the difference was statistically significant (P=0.031). The explanation for the longer survival among patients who received high opioid dose is not clear (8,9). Bercovitch et al. attributed the longer survival to a better general condition or an earlier disease stage (8). While Good et al. suggested that the longer survival is due to the earlier hospice admission of patients with complex pain syndromes that needed high opioid doses (9). Both explanations may apply to our setting. This may be supported by the finding that the highest average opioid dose was that received by patients with mesothelioma who are frequently referred to the PMU earlier in the course of their disease because of severe pain when their general condition is relatively better.
The limitations of the current study included being a retrospective one from a single center that included non-consecutive patients. In addition, we took into consideration the last prescribed opioid dose before death rather than the actual dose received because the majority of our patients died at home.
In conclusion, the use of higher doses of opioids at the end-of-life is not associated with shorter survival among patients with advanced cancer managed in an Egyptian cancer center-based PMU. Further studies to overcome barriers to cancer pain control in Egypt are mandatory.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- World Health Organization. Cancer pain relief with a guide to opioid availability. Geneva: World Health Organization, 1996.
- Dalal S, Bruera E. Access to opioid analgesics and pain relief for patients with cancer. Nat Rev Clin Oncol 2013;10:108-16. [PubMed]
- Sykes N, Thorns A. The use of opioids and sedatives at the end of life. Lancet Oncol 2003;4:312-8. [PubMed]
- Regnard C, Badger C. Opioids, sleep and the time of death. Palliat Med 1987;1:107-10.
- Bercovitch M, Waller A, Adunsky A. High dose morphine use in the hospice setting. A database survey of patient characteristics and effect on life expectancy. Cancer 1999;86:871-7. [PubMed]
- Thorns A, Sykes N. Opioid use in last week of life and implications for end-of-life decision-making. Lancet 2000;356:398-9. [PubMed]
- Morita T, Tsunoda J, Inoue S, et al. Effects of high dose opioids and sedatives on survival in terminally ill cancer patients. J Pain Symptom Manage 2001;21:282-9. [PubMed]
- Bercovitch M, Adunsky A. Patterns of high-dose morphine use in a home-care hospice service: should we be afraid of it? Cancer 2004;101:1473-7. [PubMed]
- Good PD, Ravenscroft PJ, Cavenagh J. Effects of opioids and sedatives on survival in an Australian inpatient palliative care population. Intern Med J 2005;35:512-7. [PubMed]
- Portenoy RK, Sibirceva U, Smout R, et al. Opioid use and survival at the end of life: a survey of a hospice population. J Pain Symptom Manage 2006;32:532-40. [PubMed]
- Azoulay D, Hammerman-Rozenberg R, Cialic R, et al. Increasing opioid therapy and survival in a hospice. J Am Geriatr Soc 2008;56:360-1. [PubMed]
- Bengoechea I, Gutiérrez SG, Vrotsou K, et al. Opioid use at the end of life and survival in a Hospital at Home unit. J Palliat Med 2010;13:1079-83. [PubMed]
- Radha Krishna LK, Poulose JV, Tan BS, et al. Opioid use amongst cancer patients at the end of life. Ann Acad Med Singapore 2010;39:790-7. [PubMed]
- Azoulay D, Jacobs JM, Cialic R, et al. Opioids, survival, and advanced cancer in the hospice setting. J Am Med Dir Assoc 2011;12:129-34. [PubMed]
- Alsirafy SA. Opioid consumption in Egypt. Kasr El-Aini J Clin Oncol Nucl Med 2012;8:1-3.
- Alsirafy SA. Dealing with barriers to cancer pain control in Egypt. Eur J Palliat Care 2010;17:10-1.
- Alsirafy SA. Regulations governing morphine prescription in Egypt: an urgent need for modification. J Pain Symptom Manage 2010;39:e4-6. [PubMed]
- Alsirafy SA, El-Mesidi SM, El-Sherief WA, et al. Opioid needs of patients with advanced cancer and the morphine dose-limiting law in Egypt. J Palliat Med 2011;14:51-4. [PubMed]
- Alsirafy SA, Ibrahim NY, Abou-Elela EN. Opioid consumption before and after the establishment of a palliative medicine unit in an Egyptian cancer centre. J Palliat Care 2012;28:135-40. [PubMed]
- Donner B, Zenz M, Tryba M, et al. Direct conversion from oral morphine to transdermal fentanyl: a multicenter study in patients with cancer pain. Pain 1996;64:527-34. [PubMed]
- Leppert W. Tramadol as an analgesic for mild to moderate cancer pain. Pharmacol Rep 2009;61:978-92. [PubMed]
- Alsirafy SA, El Mesidy SM, Abou-Elela EN. Where do Egyptian palliative care patients with cancer die? Am J Hosp Palliat Care 2010;27:313-5. [PubMed]