Barriers to hepatitis C virus treatment in Guangdong Province
Introduction
The hepatitis C virus (HCV) is globally prevalent, and the general population is usually susceptible to it. In 2015, the World Health Organization (WHO) estimated about 71 million people globally had chronic hepatitis C, with approximately 399,000 dying from this infection primarily due to cirrhosis and hepatocellular carcinoma (HCC) (1). China is facing the challenge of widespread liver problems. A sero-epidemiological survey in 2006 showed that about 5.6 million people in the general population were infected with HCV, while the total number of HCV carriers reached 10 million when HCV-infected persons in high-risk groups and high-incidence areas were also considered (2).
The WHO has proposed the goal of eliminating hepatitis as a public health problem by 2030, which requires 90% of those infected to be diagnosed and 80% of those diagnosed to be treated (3). At present, patients with chronic HCV infection can achieve a sustained virological response (SVR) after direct-acting antiviral agent (DAA) treatment by curing the disease, eliminating or reducing HCV-related intra- and extra-hepatic manifestations; reversing liver fibrosis; preventing progression to cirrhosis, decompensated cirrhosis, liver failure, and/or liver cancer; prolonging long-term survival; improving quality of life; and preventing the spread of HCV (4).
While DAAs, interferons, and nucleoside analogues can effectively inhibit HCV replication and improve the outcomes of patients with hepatitis C, antiviral treatment (AVT) is not feasible for all HCV-infected patients. AVT may be tailored based on its advantages and disadvantages (5). Therefore, we arranged follow-up outpatient visits for HCV-infected patients and investigated the reasons for HCV non-treatment.
Methods
Subjects
HCV-infected patients who entered the Hepatitis C Follow-up Clinic of the Third Affiliated Hospital of Sun Yat-sen University were enrolled as the subjects. The exclusion criteria included completed antiviral therapy, previous antiretroviral treatment, follow-up for less than 6 months, and lost to follow-up for more than 3 months. A total of 435 patients with chronic HCV infection entered the follow-up cohort, among whom were 248 men and 187 women aged 40.3±14.8 years.
Follow-up methods
The patients were followed up regularly by using retrospective and prospective methods. (I) The establishment of the follow-up clinic for HCV-infected persons was as follows: After the informed consent forms were voluntarily signed, the patients with chronic HCV infection were enrolled in the Hepatitis C Follow-up Clinic, where the changes of liver function before the visit, the possible infection route and time, and the interferon treatment were recorded. All patients received explanations about the purpose of AVT, drug options, treatment course, cost, adverse reactions, regular follow-up visits, etc. (II) The details of the follow-up frequency were as follows: For patients who did not receive AVT, the main cause of HCV non-treatment was recorded, and the follow-up visits continued every 6 months; patients with normal or slightly abnormal liver function were followed up every 6 months; and patients receiving interferon therapy or with severe liver dysfunction were followed up monthly or weekly depending on the disease condition. (III) The examinations during follow-up were as follows: collection of general data, testing for symptoms, and routine physical examinations were performed during the follow-up visits. The laboratory tests for liver function (ALT and AST) were performed using the Hitachi 7170 automatic biochemical analyzer. Enzyme-linked immunosorbent assay (ELISA) for anti-HCV IgM/IgG was performed by using the reagent manufactured by Shanghai Kehua Bio-engineering Co. Ltd. A quantitative polymerase chain reaction (qPCR) was performed to detect HCV RNA, and the reagent was provided by DAAN Gene Co., Ltd. of Sun Yat-sen University. Manufacturers’ instructions were strictly followed during all tests and result interpretation. Liver ultrasound was performed to identify any liver lesions.
Diagnostic criteria and antiviral treatment considerations
The diagnosis of chronic HCV infection was based on the Chinese Guidelines on the Prevention and Treatment of Hepatitis C (2001 and 2004 editions) released by the Chinese Medical Association Liver Disease Society (6,7). The indication for AVT for chronic HCV infection is patients who have had a positive HCV RNA assay. HCV nontreatment refers to patients who are eligible for AVT but have decided not to receive AVT despite it being recommended by physicians at least three times, each time more than three months apart, or patients whose conditions are not indicated for AVT.
Statistical analysis
Statistical methods including Chi square test, t-test, homogeneity analysis of variance, and linear correlation analysis were performed in the SPSS 16.0 statistical software package. A P value of <0.05 was considered significantly different.
Results
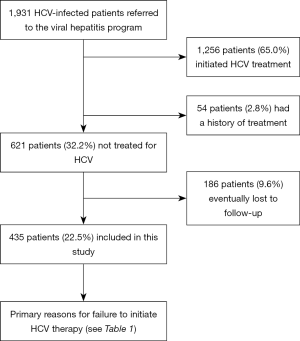
A total of 1,931 HCV-infected patients were admitted to the Hepatitis C Follow-up Clinic, among whom 1,256 patients (65%) completed AVT, 54 (2.8%) had a history of AVT, and 621 (32.2%) did not receive AVT. Among them, 186 patients (9.6%) were lost to follow-up. In total, 435 patients (22.5%) entered the final analysis. The case enrollment algorithm is shown in Figure 1.
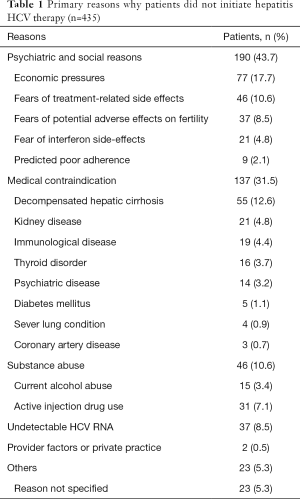
Reasons for HCV nontreatment: Of the 1,931 patients, 435 (22.5%) did not receive AVT, the main reasons for which are summarized in Table 1. Among them, the virus load was persistently negative in 37 patients (8.5%). In the remaining 398 patients (91.5%), HCV RNA repeatedly tested positive. The causes of nontreatment in these patients included economic pressures (n=77, 17.7%), old age or fears of treatment-related side effects or uncertainty (n=46, 10.6%), fear of potential adverse effects on fertility (n=37, 8.5%), fear of interferon side-effects (n=21, 4.8%), and dosing inconvenience during study or work (n=9, 2.1%). In addition, 137 patients (31.5%) had medical contraindications including decompensated hepatic cirrhosis (n=55, 12.6%), uncontrolled autoimmune diseases (e.g., autoimmune hepatitis and systemic lupus erythematosus) (n=19, 4.4%), renal dysfunction (n=21, 4.8%), thyroid disease (hyperthyroidism) (n=16, 3.7%), depression (n=14, 3.2%), uncontrolled diabetes (n=5, 1.1%), severe lung disease (active tuberculosis) (n=4, 0.9%), symptomatic heart disease (n=3, 0.7%), alcoholism (n=15, 3.4%), drug addiction (n=31, 7.1%), lack of physician recommendation (n=2, 0.5%), and unknown reasons (n=23, 5.3%).
Full table
Discussion
The role of AVT in treating chronic HCV infection has been well recognized due to its high cure rate and short treatment course. Several studies have shown that AVT can effectively reduce the HCV load and improve liver function, thus decreasing the risk of cirrhosis and liver cancer (8). However, a large proportion of patients with chronic HCV infection fail to receive AVT in real-world practice.
Our current follow-up data showed that the rate of AVT was only 65% in patients with confirmed HCV infection. In a study from abroad, only 25% of HCV-infected patients were diagnosed due to the occult nature of this condition, and about 50% of these patients went to hospital; among the patients who sought help at hospital, only 1.1–30% received AVT (1,9). The high AVT rate in our series might be explained by two factors: first, the indications of AVT were strictly applied in our series, and all the subjects were well informed about the therapeutic goals, drug selection, treatment course, cost, and adverse reactions; and second, the patients were followed up in a teaching hospital where the AVT rate is higher than ordinary hospitals (similarly, McLaren et al. found that the AVT rate in a teaching hospital was 30 times that of ordinary hospitals (2,10).
There are many spatial and temporal barriers to AVT. Mclaren et al. analyzed patients with HIV-HCV co-infection and found that the ACT rate was 12–34%. The key primary reasons included poor compliance (23–40%), alcoholism or drug abuse (21–26%), uncontrolled mental illness (8–21%), decompensated cirrhosis or advanced liver disease (3–12%), HIV progression (13%), and co-existence of other diseases (8–24%) (2,3,10,11). Of the 435 patients followed up in our current study, psychosocial factors (accounting for 43.7%) were the main barriers to non-antiviral treatment, of which economic factors accounted for 17.7%, followed by fears of safety and uncertainty (10.6%), fears of side effects (4.8%), and fears of potential adverse effect on fertility (8.5%). Evon found that the psychosocial barriers in the United States mainly included fear of being discriminated against (26–50%), poor compliance (24–57%), lack of medical insurance (10%), and being unemployed and unmarried (12%) (1,4,9,12). Despite the economic boom in China in the past decades, the per capita income is uneven, and most of the current antiviral drugs have not been listed in medical insurance programs. Many patients ultimately forego AVT because they cannot afford its cost. The subjects followed up in our current study were from the relatively rich Pearl River Delta region, and still up to 17.7% of patients failed to continue AVT due to economic considerations, suggesting AVT is still unaffordable for some patients. This is a call for the development of cheaper and more effective anti-HCV drugs; meanwhile, the government should include more antiviral drugs in the medical insurance programs and decrease the drug prices. In the clinical settings, the efficacy of AVT should be balanced with the patient’s economic status. In addition, research has shown that drug-related side effects were feared twice as much (up to 67% of patients) as the asymptomatic natural disease course among patients (1,9). In our current study, 104 patients (23.9%) did not receive AVT due to fear of AVT-related side effects. AVT interferes with the natural course of HCV infection, but each patient may suffer from different treatment-related side effects, which, to a certain degree, affect the quality of life and may even lead to drug discontinuation or withdrawal. Therefore, good physician-patient communication is required to increase the awareness and knowledge of patients of both HCV and AVT, build treatment confidence, and eliminate their fears and concerns before the initiation of AVT.
AVT was not initiated in 46 of our patients due to drug abuse. In the United States, injection drug users (IDUs) are a major cause of delayed ACT for HCV: up to 60% of patients with chronic hepatitis C are also IDUs; IDUs often have mental disorders and poor compliance and thus are more susceptible to the side effects of ACT. Therefore, abstinence from drugs is required for at least 1 year before the initiation of AVT (5,8).
During our follow-up visits, 31.5% of patients failed to receive AVT due to the presence of contraindications. Previous studies have shown that mental disorders are aggravated after treatment with interferon in 21–58% of HCV patients with underlying mental diseases (5,8). Therefore, special attention should be paid to the side effects of interferon and ribavirin in HCV patients with accompanying underlying diseases, and individualized treatment should be carried out after these diseases are actively controlled.
In addition, 9 patients in our current study did not receive AVT due to poor compliance. Butt et al. also found that, among veterans, 24–57% of AVT-naive patients did not undergo further diagnosis and treatment mainly due to poor compliance, which was associated with a low education background, alcoholism, and drug abuse (6,13).
In summary, the low rate of AVT is associated with a variety of factors including high treatment cost, low diagnostic rate, lack of relevant knowledge, and economic burden. In Guangdong, HCV patients do not receive AVT mainly due to economic constraints, patients' fear of side effects, and the presence of contraindications.
Our current study had certain limitations. The subjects in this study were patients who were treated in a teaching hospital; these patients had more severe liver damage and were more willing to receive AVT, and it was more feasible to do so than in the general HCV population of community hospitals.
Acknowledgments
We would like to express our thanks to the doctors (Jianyun Zhu, Fangfang Wei, Zhishuo Mo) of the Department of Infectious Diseases, the Third Affiliated Hospital of Sun Yat-sen University in Guangzhou, China, for collecting clinical data and throat swab specimens.
Funding: Optimized treatment and establishment of standardized follow-up system of chronic HCV infection, and early diagnosis of HCV-related liver cancer in Guangdong Province (2014B020212025).
Footnote
Conflicts of interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University. The ethical approval number was [2018]02-305-02. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva: World Health Organization; 2018.
- Chen YS, Li L, Cui FQ, et al. A sero-epidemiological study on hepatitis C in China. Zhonghua Liu Xing Bing Xue Za Zhi 2011;32:888-91. [PubMed]
- Seale A, Broutet N, Narasimhan M. Assessing process, content, and politics in developing the global health sector strategy on sexually transmitted infections 2016-2021: Implementation opportunities for policymakers. PLoS Med 2017;14:e1002330. [Crossref] [PubMed]
- European Association for the Study of the Liver; European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol 2018;69:461-511. [Crossref] [PubMed]
- Evon DM, Golin CE, Stoica T, et al. What's Important to the Patient? Informational Needs of Patients Making Decisions About Hepatitis C Treatment. Patient 2017;10:335-44. [Crossref] [PubMed]
- Chinese Society of Infectious Diseases and Parasitology Csoh. Prevention and treatment of viral hepatitis. Chinese Journal of Infectious Diseases 2001;19:56-62.
- Society of Liver Diseases and Society of Infections. Guidelines for the prevention and treatment of hepatitis C. Chinese Journal of Internal Medicine 2004;43:551-5.
- Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335-74. [Crossref] [PubMed]
- McGowan CE, Fried MW. Barriers to hepatitis C treatment. Liver Int 2012;32 Suppl 1:151-6. [Crossref] [PubMed]
- McLaren M, Garber G, Cooper C. Barriers to hepatitis C virus treatment in a Canadian HIV-hepatitis C virus coinfection tertiary care clinic. Can J Gastroenterol 2008;22:133-7. [Crossref] [PubMed]
- Restrepo A, Johnson TC, Widjaja D, et al. The rate of treatment of chronic hepatitis C in patients co-infected with HIV in an urban medical centre. J Viral Hepat 2005;12:86-90. [Crossref] [PubMed]
- Evon DM, Simpson KM, Esserman D, et al. Barriers to accessing care in patients with chronic hepatitis C: the impact of depression. Aliment Pharmacol Ther 2010;32:1163-73. [Crossref] [PubMed]
- Butt AA, Wagener M, Shakil AO, et al. Reasons for non-treatment of hepatitis C in veterans in care. J Viral Hepat 2005;12:81-5. [Crossref] [PubMed]