Advances in loco-regional palliation of unresectable cholangiocarcinomas
Introduction
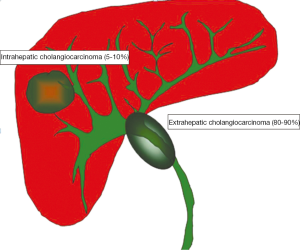
In the United States, cholangiocarcinoma (CC) is a relatively rare form of cancer with an annual incidence of 1-2 cases per 100,000 individuals and approximately 2,000 to 3,000 new cases per year (1). Advanced age, cigarette smoking and primary sclerosing cholangitis (2) are the main risk factors for CC in western countries. On the other hand, chronic parasitic infestations of the biliary system and intra-hepatic biliary stones are the main risk factors for CC in Asia where the prevalence of CC is higher (3). The most common type of CC is the extrahepatic type (extrahepatic cholangiocarcinoma, ECC) which includes cancers involving the confluence of the right and left hepatic ducts (80% to 90%). The intrahepatic CC (ICC) consists of 5% to 10% of all CC. The anatomic margins for distinguishing intra- and extrahepatic CC are the second order bile ducts (Figure 1).
Diagnosis
The diagnosis of CC can be challenging due to the fact that most of patients develop symptoms only when the tumor is in advanced stage. Diagnosis requires a high index of suspicion and often multiple tests including laboratory, endoscopic and cross sectional imaging studies. Computer tomography (CT) and magnetic resonance imaging (MRI) are important as they provide information about the tumour size, location, presence of vascular invasion, lymph node involvement and metastases (4). A summary of the most used diagnostic modalities for CC is reported in Table 1. Although in some circumstances it might be difficult for the location or for the presence of other clinical conditions, accurate staging and histological or cytological diagnosis is always recommended prior to initiation of palliative therapies for CC.

Full table
Intrahepatic cholangiocarcinoma
Multiple studies have documented a steady increase in the incidence of ICC over the past several decades; notably in North America, Europe, Asia, and Australia (5). Although the actual reasons for this increase are unclear; advances in the diagnostic methods may be partially responsible for this. ICC arises from the bile ducts inside the liver and accounts for 5% to 10% of all CC. Most of the times, ICC presents as a solid mass in the liver with possible infiltration of the portal vein pedicles, hepatic veins and bile ducts. It is usually diagnosed at an advanced stage when patients develop obstructive jaundice, malaise, weight loss and abdominal discomfort (6). The average age at presentation is the seventh decade of life with male to female ratio of 1.5. Surgical resection is the only potential curative therapy that is feasible in 46% to 75% of patients (7). Tumor size, lymph node status, presence of intrahepatic metastasis and vascular invasion are important prognostic parameters. Overall, 5-year survival rate is lower than 5%. Local recurrence is the most frequent problem after surgery.
Extrahepatic cholangiocarcinoma
ECC arises from bile ducts within the hepato-duodenal ligament. ECC comprises 90-95% of all CC cases (8). These tumors frequently present with obstructive jaundice and most of them are unresectable (9). Even after radical resection, the prognosis of ECC is poor with 5-year survival rate as low as 10% (10).
Palliation of intrahepatic cholangiocarcinoma
Radiofrequency ablation (RFA)
RFA represents a modern method for the palliation of ICC. Although the technique may differ from centre to centre, it requires the insertion of a needle into the tumor mass that can be performed percutaneously or intra-operatively with the guidance of an ultrasound machine or computerized tomography (11). A high frequency electromagnetic wave is emitted for 12-15 minutes using a 200-W generator set to deliver maximum power under automatic impedance control. The generated heat leads to coagulation necrosis of the tumor.
RFA has the advantage of being a safe procedure that can be tolerated well even by patients who have several comorbidities as it can be performed under sedation, without the need for a laparotomy. Nevertheless, it is usually indicated for lesions measuring less than 5 cm in size as the effectiveness of RFA is inversely related to the diameter of the tumors. In addition, the effect of RFA is reduced when tumors are located in proximity of large vascular structures because the blood flowing in their lumen keeps the temperature of tumor cells from reaching the critical temperature necessary for their necrosis.
Kim et al. (12) treated 13 patients with different ICC sizes. Among these 13 patients, 9 were deemed unresectable since they had poor hepatic reserve due to liver cirrhosis. The 5-year survival rate was 15% despite the fact that two large lesions (7 and 8 cm) were not adequately ablated. They concluded that survival rate after RFA was comparable with surgical resection and that RFA provided successful local tumor control in patients with intermediate (3-5 cm) and small (≤3 cm) ICC.
Another single center experience (13) reported a higher 5-year survival rate (83.3%). The largest series regarding RF ablation included 17 patients with 26 ICCs. Transarterial chemoembolization (TACE) before RF ablation was used to treat 7 patients. The median tumor diameter at the time of RFA was 4.4 cm (2.1-6.8 cm). The 5-year overall survival rate was 28.9% with an overall complication rate of 3.6% (14) that included liver abscess, hematoma and post-ablation syndrome (abdominal pain, nausea, vomiting and fever).
Role of transcatheter intraarterial therapies
Transcatheter intraarterial therapies are widely used for primary and secondary hepatic malignancies with favorable outcomes (15). The advantages of these therapies are their low toxicity profiles and minimal invasiveness. The basic principle common of all intra-arterial therapies is to selectively deliver cytotoxic agents in the arteries supplying blood to the tumor. These therapies include: transarterial embolization, intraarterial chemoinfusion, TACE with or without drug eluting beads and radioembolization (RE) with use of yttrium 90. However, their use for CC is not well-established. Trials investigating the role of TACE and RE in the management of unresectable CC have only recently emerged in the literature (16,17).
One of the largest series was reported by Gusani et al. (18) and included 42 patients. All of the patients had preserved liver function and acceptable performance status. In case of biliary obstruction a decompressive biliary stent was placed prior to the procedure. TACE was carried out by cannulation of the femoral artery, advancement of the arterial catheter to right or left hepatic artery under fluoroscopy and subsequent chemoinfusion (Gemcitabine or combination of Gemcitabine with cispaltin or oxaliplatin). Embolization was accomplished after administration of the chemotherapeutic drugs to the tumor. Median tumor size was 9.8 cm (range, 13.3-17 cm). Minimal extrahepatic disease was present in 19 patients at the time of the treatment. The median overall survival was 9.1 months and patients with ICC were found to have a better median survival compared to hilar tumors (P=0.017).
Another study analyzed the patients with unresectable ICC undergoing TACE (n=72) or best supportive therapy alone (e.g., pain control, biliary drainage) (n=83). The former group survived a median of 12.2 months while the latter survived a median of 3.3 months (19). Among 72 patients whom underwent TACE, 9 sustained hematological toxicities (hemoglobinemia, thrombocytopenia, neutropenia) while 25 suffered non-hematological side effects. The most frequent side effect was post-embolization syndrome which presented with minor abdominal pain, fever, nausea/vomiting and fatigue that resolved within two to three days.
Kuhlman and colleagues (20) recently reported encouraging results regarding TACE using irinotecan-eluting beads (iDEB-TACE). This study included 26 patients treated with iDEB-TACE (200 mg irinotecan), 10 patients with conventional TACE using 15 mg mitomycin C mixed with lipiodol and 31 patients with systemic gemcitabine and oxaliplatin. The overall survival rate of the patients in the iDEB-TACE group was six months longer than the conventional TACE group. Drug eluting beads loaded with doxorubicin (DEBDOX) were found to be effective in another study, paving the way for combination of chemotherapeutic drug treatments with this technique (21).
Role of radioembolization
RE with yttrium-90 (90Y) microspheres has been used in patients with hepatocellular carcinoma or metastatic liver tumors. However, there are limited data regarding the safety and efficacy of RE in patients with ICC (16,22-25).
90Y is a pure beta-emitter with a mean tissue penetration of 2.5 mm and half-life of 2.6 days and it can be applied in outpatient setting (16). The principle of RE is based on the preferential vascular distribution of radioactive 90Y microspheres to the tumor vasculature. This allows delivery of high radiation doses with relative sparing of normal liver tissue (16). It can be administered as single or in multiple sessions according to the tumor burden (24).
Initially, an arteriography is performed and extrahepatic branches arising from the hepatic arteries are embolized to prevent extra-hepatic escape of the microspheres. 90Y microspheres are then injected under intermittent fluoroscopic visualization along with alternating contrast medium injections to check the anterograde hepatic arterial flow (24). The procedure is terminated when the previously calculated dose is injected or vessel stasis is detected. A single photon emission computed tomography scan is performed to confirm the target deposition of 90Y, 24 hours after treatment (16,23).
Rafi et al. (24) reported a prospective cohort study including 19 patients with chemotherapy refractory ICC. Their results indicated that unlike RF ablation, 90Y RE can be effective for management of large size tumors (≥5 cm) (24). Ibrahim et al. reported that they treated 24 patients who were referred for RE after failure of systemic chemotherapy. The median survival in this group of patients was 4.4 months (22,25). Subsequently, they reported a larger series which supported the former findings (25): 5 out of 46 patients were down-staged with RE and were deemed surgically resectable. All patients in this subset were treatment naïve and were considered locally advanced at time of diagnosis (e.g., central tumor abutting right and left hepatic veins).The median survival rate was 14.6 months for patients with solitary tumors (n=29) (25). One of the most common complications of RE is post-RE syndrome (PRS). Clinical manifestations such as fatigue, nausea, vomiting, anorexia, fever, abdominal discomfort and cachexia can be present in 20-55% of cases (16,26). Prophylaxis with steroids and antiemetic agents was recommended for prevention of PRS.
Although less frequent (17-20%), hepatic dysfunction, biliary stricture, cholecystitis, portal hypertension, radiation pneumonitis, gastrointestinal ulcers, vascular injury and lymphopenia are the other potential complications of RE (26). No procedure-related mortality has been reported (16,24).
Role of stereotactic body radiation therapy (SBRT)
SBRT is an external radiation treatment modality by which high dose radiation is administered in millimetric accuracy to reduce damage to tissues surrounding the target tumor. The patient is positioned and then immobilized during the procedure so that radiation can be focused precisely to the tumor (27).
Immobilization of the patient is important in order to provide accuracy and repetition of the treatment. It is provided by a customized vacuum pillow fixed in a stereotactic body frame. The body frame incorporates an external coordinate system that is visible on CT. This system establishes a reference target volume by contouring the gross tumor volume on the CT images. In order to clearly define the gross tumor volume and recognize the biliary system elements, an intraluminal stent can be placed in the hepatic ducts before the procedure. The gross tumor volume, together with the areas of diagnostic uncertainty constitutes the clinical target volume (28). Radiation (30-60 Gy) is usually delivered with five to eight coplanar or non-coplanar beams formed by a multileaf collimator (29). Patients are treated with either three or five fractions on consecutive days, with an effort to complete the therapy in one week (29).
Some early reports compared SBRT with external beam radiation therapy in patients with unresectable CC (27,29). These studies have shown that SBRT provides better local tumor control but has higher toxicity.
Kopek et al. (27) reported the largest series which included 27 patients with unresectable CC. These authors used SBRT with 45 Gy delivered in three fractions. They recorded a 1-year local tumor control rate of 84% and median overall survival of 10.6 months. The most frequent site of first progression was reported outside the treated volume. During post-procedure follow-up, six patients developed duodenal ulcerations while three patients sustained duodenal stenosis. They noticed that the mean maximum SBRT dose corresponding to 1 cc of duodenum was significantly higher in patients developing duodenal ulceration or stenosis. Hence they recommended limiting radiation to less than 21 Gy in 3 fractions per 1 cc duodenum. In addition, they suggested use of more sophisticated image guiding techniques (e.g., Cyber-Knife) to minimize duodenal radiation exposure.
Barney et al. (29) included 10 CC patients with 12 lesions. Among these lesions, 6 were unresectable primary tumors while the other 6 were post-surgery recurrent lesions. Systemic chemotherapy was given to four patients while one patient received EBRT before undergoing SBRT. The median prescribed dose was 55 Gy in 5 fractions. During follow-up time (2-26 months), no local recurrence was detected. However, four patients had recurrence in a distant portion of the liver. The one year estimated overall survival rate was 73%. Early toxicity reactions were encountered in 90% of patients. They were ‘low-grade’ reactions such as mild nausea and fatigue. On the other hand, late toxicity reactions were less frequent but ‘high-grade’ such as biliary stenosis requiring stent placement which was diagnosed in 1 patient during follow-up.
Polistina and colleagues (28) treated ten unresectable CCA patients with SBRT plus gemcitabine. For SBRT application, they used Cyber-Knife which is a frameless, image guided, robotic, radiotherapy device. They have given 30 Gy SBRT in 3 fractions. The overall local response rate was 80% and median survival was found as 35.5 months (range, 8-51 months). Their high disease control rate was not complicated by a high toxicity rate; the overall toxicity rate was 30% with no ‘high-grade’ toxicity. They stated that SBRT is effective in improving local control of the disease without any or with minimal effect on progression to metastatic disease and thus it should be combined with chemotherapy in order to achieve better systemic control.
Implementation of effective local disease control and non-invasiveness gives SBRT the potential to be an alternative or supplemental treatment modality for patients with locally advanced CC. However, late toxicity reactions still remain as a source of concern. In addition, its role in multimodality treatment setting is yet to be identified by further studies.
Role of intrahepatic stent placement by ERCP
Endoscopic retrograde cholangiopancreatography (ERCP) guided biliary drainage is occasionally performed in the setting of ICC because of its proximal location. In general, the more proximal the CC and the more multifocal the disease, the more likely the patient is to require percutaneous therapy/drainage rather than endoscopic drainage. Endoscopic biliary drainage and subsequent stent placement is more suitable for palliation of hilar obstructions. It is generally considered as less invasive than percutaneous drainage since it does not require creation of a transhepatic tract (30). There are two types of Endoscopic biliary drainage, endoscopic nasobiliary drainage and endoscopic retrograde biliary drainage. Nasobiliary drainage is an endoscopic procedure which uses a 250-cm long polyethylene tube placed above the stenosis to provide outflow of the bile (31). Since the external tube gives discomfort to the patients, this approach has become obsolete.
On the other hand, endoscopic retrograde biliary drainage offers the advantages of physiological bile drainage and increased patient comfort. Thus, it is the preferred choice for pallia¬tion of patients with hilar CC or extra-hepatic CC (32).
Role of percutaneous transhepatic drainage
Percutaneous biliary drainage may be required either during the preoperative period (for surgical candidates) or for palliative reasons in patients who will not undergo surgical resection (33). Regardless of the indication, percutaneous trans-hepatic cholangiography and subsequent percutaneous biliary drainage can usually be performed under sedation. A small skin incision is made; a 22-gauge Chiba needle is advanced into the liver usually towards the 9th or 10th intercostal space (34). The probe is then removed and the needle is slowly withdrawn while injecting small amounts of contrast to opacify the biliary tree. Once the needle is confirmed to be within a biliary duct, a limited cholangiogram is obtained to see and confirm the target biliary segment based on the preprocedural planning. Then a wire is advanced to the biliary tree. The operator may elect to slide an external biliary drainage catheter over the wire and connect it to bag drainage if the patient is hemodynamically unstable (34). However, if the patient is stable, the operator should attempt to advance the wire into the small bowel and place an internal-external biliary drainage catheter (34).
In the palliative treatment setting, percutaneous transhepatic drainage is a highly effective procedure with reported technical success rates of 95-100% and clinical success rates of 70-80% (33,35). In one study, 57% of patients had a significant reduction in their serum bilirubin following drainage, with 44% experiencing a reduction to less than 2 mg/dL (33). The average period for bilirubin to drop to levels less than 2 mg/dL was 2.8 weeks. Pruritis was reported to improve in 1 to 4 weeks. Relief of biliary obstruction, in addition to symptom relief, is also correlated with improved overall survival (33,35). Minor complications of the procedure are post-procedure pain, catheter leaks and fever and they are encountered in 31% of patients (33,35). The most common major complication is cholangitis which is detected in 7.8% of the patients (33). Transient hemobilia is a relatively common finding after the procedure however less than 2% of patients may experience significant bleeding that requires transfusion. As a late complication, catheter dysfunction is frequent and is seen in up to 47% of patients. Catheter dislodgement has been reported in 8-17% of patients (33,35).
In cases of proximal lesions or in patients with multiple strictures percutaneous drainage may be superior to ERCP since it can target the strictures in the biliary tract individually.
Palliation of extrahepatic cholangiocarcinoma and Klatskin tumors
Endoscopic palliation
Endoscopic retrograde biliary drainage with potential stent placement is a desirable method for palliation of malignant extrahepatic obstruction. Endoscopic stents can be either self-expanding metallic or plastic (polyethylene). Metallic stents are more expensive than plastic stents but they have larger diameters (30 vs. 11.5 F) and therefore provide longer patency rates (36).
The major drawback of endoscopic stenting is that the patency of endoscopic stent can be limited by tumor overgrowth, biofilm deposition, biliary sludge or granulation tissue formation. These characteristics may result in ongoing or recurrent biliary obstructions that require re-intervention in approximately 30% of patients (37,38).
Plastic stents often need to be changed in 2 to 3-month intervals, while metallic stents can remain for nine months (39,40). Several randomized controlled studies have compared metallic stents with plastic stents in patients with inoperable malignant biliary obstruction (41-43). These studies concluded that metallic stents were more cost effective for patients who were expected to survive for longer than six months as they required less re-interventions and shorter hospitalizations (41,42).
Drainage of 25-50% of the hepatic parenchyma is usually sufficient for adequate palliation of obstructive jaundice in the absence of cholangitis (44). A randomized controlled study including 157 patients compared unilateral stenting versus bilateral stenting in patients with malignant hilar obstruction and found that unilateral drainage was sufficient to relieve obstruction with no difference in complications and overall survival (45).
Bilateral or multiple stenting is indicated exclusively for patients with cholangitis of the contralateral lobe or segmental ducts (46).
Role of percutaneous transhepatic drainage
Percutaneous drainage of the biliary system is necessary to palliate obstructive jaundice when endoscopic drainage fails, or when the endoscopic operator is inexperienced. An additional use of percutaneous technique is the Rendezvous procedure, where the percutaneously placed catheter guides the endoscopist to stent a biliary stricture that could not be palliated by ERCP alone (47). For most patients, the ultimate goal of the percutaneous drainage procedure is the insertion of an internal/external drainage catheter or an internal stent. Internal stents are less prone to dislodgement and malfunction than percutaneous drainage catheters. Their median patency rate is five to six months (48). A recent multicenter study has shown that the placement of percutaneous self-expanding metallic stents across a hilar CC is associated with a higher success rate and lower risk of procedure-induced cholangitis (49) than endoscopic polyethylene endoprosthesis. Frequently, hilar tumors do not involve the three major hilar ducts (left hepatic, right anterior sectoral hepatic and right posterior sectoral hepatic) and two or more stents must be placed for adequate drainage.
Role of intraluminal radiation therapy
There are three types of radiation therapy: external, brachytherapy and the combination of both. In prospective trials, external radiation therapy alone did not give any encouraging results for the palliation of CC. On the other hand, the luminal access achieved by endoscopic or percutaneous route has stimulated interest in the application of intraluminal radiation (brachytherapy). The key advantage of brachytherapy is the focal delivery of high radiation doses from a short distance which helps in avoiding hepatotoxicity. This can be performed by trans-duodenal approach or by percutaneous technique. Initially, location and length of the malignant bile duct stricture is identified by cholangiography. Then, Iridium 192 (Ir192) seeds mounted on a catheter are placed directly across the stricture (50). Brachytherapy is applied to a 1 cm radius at a dose and rate based on two pre-determined regimens. Low-dose brachytherapy involves administering 30-45 Gy over 24-60 hours. Patients require hospitalization while the radioactive seeds are in place to avoid radiation exposure to others. In contrast, high-dose brachytherapy may be performed as an outpatient and a total dose of 24-30 Gy can be given in four fractions separated by at least 6-hour intervals. Treatments are usually given over two days. Both high-dose and low-dose regimens provide similar outcomes (36).
In addition, 5-flourouracil is often given concomitantly for tumor radiosensitization (51). Following brachytherapy, plastic or metallic stents are placed at the treatment site to prevent biliary obstruction due to edema. Combination of external radiotherapy with intra-luminal brachytherapy was also reported (52-54). With this method a total dose of 20-30 Gy is given in 2-3 fractions in addition to external radiotherapy. Higher total radiation dose provided a survival advantage; however, the high incidence of complications such as stenosis, upper gastro-intestinal bleeding, portal vein obstruction and ascites limited its use (47,52). The palliative effect of intraluminal brachytherapy in the setting of CC was previously analyzed (55). Its ability to maintain ductal patency was suggested to delay biliary obstruction and increase survival. However, the survival increase was not statistically significant. The low patient number and the wide tumor stage range might have contributed to the statistical insignificance. Authors from various centers admit that brachytherapy is expensive as a method of palliation. It requires several days of hospitalization with expensive radioactive sources (56). The staff involved may require additional training on brachytherapy. In case of complications such as cholangitis, re-admissions may be needed. The cost per patient is estimated to be thousands of dollars with only marginal improvement promise on survival. Thus, use of this technique should be studied further with prospective controlled clinical trials.
Role of intraluminal phototherapy
Photodynamic therapy (PDT) is a relatively new technique. It is given for local control of unresectable tumors in patients without distant metastases. It is a two-step procedure: the first step entails the infusion of a photosensitizing drug that accumulates selectively in tumoral cells 24-48 h after its administration. Subsequently, endoscopic retrograde cholangiography is performed in order to define the anatomic distribution of malignant tissue and the extent of the disease within the biliary ducts. Bougie and balloon dilatation of the malignant strictures is performed to facilitate the diffuser placement (57). The diffuser probe is placed through a stent or alternatively through a single operator choledoscope (Spyglass®) and photo-activation is performed by light.
Photo-activation of the compound transfers the energy to molecular oxygen generating cytotoxic free radicals which lead to tumor necrosis to a depth of 4 to 6 mm. One or more segments can be treated at once. In cases where treatment by endoscopic route is not feasible, percutaneous biliary access can be used to deliver PDT.
Photosensitizers used for photodynamic treatment of CC are either hematoporphyrin or chlorine derivatives which all have high tissue penetration power. However, only porfimer sodium (Photofrin®) is FDA-approved for PDT. After PDT, patients should be kept in a darkened room for 3-4 days in order to eliminate the photosensitivity reactions.
A recent prospective randomized study done by Ortner et al. included 39 patients with stage III or IV CC. These patients underwent biliary stent insertion with or without PDT. Overall survival was longer in the group treated with PDT (493 vs. 98 days). In addition, the rate of cholestasis was lower while their quality of life was higher (56). The authors concluded that PDT can be well tolerated even for severely ill patients.
Witzigmann et al. (9) reported that median survival of hilar CC patients with R1-2 surgical resection was similar to median survival of hilar CC cases that underwent PDT plus stenting. This study included 184 cases, 42 of which received R0 resection. R1-2 resection was performed in 18 cases while 56 patients were treated with biliary stent and 68 with biliary stent insertion and PDT. Patients who underwent stent insertion and PDT had a median survival of 12 months. On the other hand, median survival was significantly lower (6.4 months) in the patient group treated with biliary stent insertion alone. PDT preceding surgical resection of the tumor (i.e., neoadjuant PDT) was proposed and applied for the first time by these authors. In the context of this new approach, PDT was followed by the surgical resection of the tumor after a median period of 3.4 weeks. Although they are all promising, these preliminary results require further verification before PDT can be considered as a viable palliative treatment option in the setting of CC.
Role of intraluminal radiofrequency ablation
RFA produces a high frequency electric current which ultimately leads to coagulation necrosis of neoplastic cells. This technique requires a bipolar RF catheter which is placed through the malignant biliary stricture under fluoroscopic guidance. The catheter has two ring electrodes 8 mm apart with the distal electrode at 5 mm distance from the leading edge, providing local coagulative necrosis over a 2.5 cm length. Alis et al. (58) applied RF ablation sessions lasting for 2 minutes including 15 seconds breaks. This one-armed study included 17 patients with either unresectable CCs or high risk comorbidities that prevented surgical therapy. After completion of RF sessions a metallic stent was placed in the biliary duct to prevent obstructive jaundice. The median duration of stent patency was reported as 9 months (range, 6-15 months) and most of them (9 out of 10) did not require re-intervention. They concluded that endobiliary RF ablation therapy was an acceptable palliative treatment modality for extrahepatic CC.
Steel et al. (59) performed endoscopic RF ablation in 22 patients, 6 of whom had unresectable CC. After completing RFA, a self-expandable metallic stent was placed through the intervened strictures. This study did not include long term follow up regarding biliary patency but 16 out of 22 stents were still patent 90 days after the intervention.
Conclusions
Surgical resection remains the only potential cure for patients affected by CC. Despite significant advances in diagnostic modalities, the vast majority of patients present with metastases or with advanced locoregional disease that prevents surgical therapy. Even for patients who undergo surgical treatment, recurrent disease is common and palliative interventions are often necessary. The main goals for the palliation of patients with advanced CC are decompression of the biliary system and control of the tumor growth.
Biliary decompression can be reached by both endoscopic and percutaneous drainage. Endoscopic biliary decompression is favored as it is better tolerated and provides a more physiologic biliary drainage. Percutaneous drainage is usually indicated when the biliary tree cannot be stented endoscopically. A multidisciplinary approach is often the best strategy to select the optimal option for these patients and maximize their comfort and survival.
In recent years, brachytherapy, PDT, endoscopic and percutaneous ablation and trans-arterial chemo and radiotherapy have been introduced in the armamentarium of physicians. Although the evidence for their use is still quite limited, recent studies have shown promising results. Yet, cost-effective analyses are not available and will be necessary before their large implementation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology 2004;66:167-79. [PubMed]
- Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis 1994;14:109-14. [PubMed]
- Haswell-Elkins MR, Mairiang E, Mairiang P, et al. Cross-sectional study of Opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int J Cancer 1994;59:505-9. [PubMed]
- Vasilieva LE, Papadhimitriou SI, Dourakis SP. Modern diagnostic approaches to cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2012;11:349-59. [PubMed]
- Tillich M, Mischinger HJ, Preisegger KH, et al. Multiphasic helical CT in diagnosis and staging of hilar cholangiocarcinoma. AJR Am J Roentgenol 1998;171:651-8. [PubMed]
- Shariff MI, Khan SA, Westaby D. The palliation of cholangiocarcinoma. Curr Opin Support Palliat Care 2013;7:168-74. [PubMed]
- Nakagohri T, Kinoshita T, Konishi M, et al. Surgical outcome and prognostic factors in intrahepatic cholangiocarcinoma. World J Surg 2008;32:2675-80. [PubMed]
- Krokidis M, Fanelli F, Orgera G, et al. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovasc Intervent Radiol 2010;33:97-106. [PubMed]
- Witzigmann H, Berr F, Ringel U, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg 2006;244:230-9. [PubMed]
- Mattiucci GC, Autorino R, D’Agostino GR, et al. Chemoradiation and brachytherapy in extrahepatic bile duct carcinoma. Crit Rev Oncol Hematol 2014;90:58-67. [PubMed]
- Haidu M, Dobrozemsky G, Schullian P, et al. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Intervent Radiol 2012;35:1074-82. [PubMed]
- Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 2011;196:W205-9. [PubMed]
- Giorgio A, Calisti G. Radiofrequency ablation for intrahepatic cholangiocarcinoma: retrospective analysis of a single centre experience. Anticancer Res 2011;31:4575-80. [PubMed]
- Fu Y, Yang W, Wu W, et al. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol 2012;23:642-9. [PubMed]
- Lewandowski RJ, Geschwind JF, Liapi E, et al. Transcatheter intraarterial therapies: rationale and overview. Radiology 2011;259:641-57. [PubMed]
- Hoffmann RT, Paprottka PM, Schon A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol 2012;35:105-16. [PubMed]
- Ray CE Jr, Edwards A, Smith MT, et al. Metaanalysis of survival, complications, and imaging response following chemotherapy-based transarterial therapy in patients with unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol 2013;24:1218-26. [PubMed]
- Gusani NJ, Balaa FK, Steel JL, et al. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg 2008;12:129-37. [PubMed]
- Park SY, Kim JH, Yoon HJ, et al. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol 2011;66:322-8. [PubMed]
- Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol 2012;24:437-43. [PubMed]
- Schiffman SC, Metzger T, Dubel G, et al. Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survival. Ann Surg Oncol 2011;18:431-8. [PubMed]
- Ibrahim SM, Mulcahy MF, Lewandowski RJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres results from a pilot study. Cancer 2008;113:2119-28. [PubMed]
- Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 2010;17:484-91. [PubMed]
- Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol 2013;36:440-8. [PubMed]
- Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol 2013;24:1227-34. [PubMed]
- Riaz A, Lewandowski RJ, Kulik LM, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol 2009;20:1121-30. [PubMed]
- Kopek N, Holt MI, Hansen AT, et al. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol 2010;94:47-52. [PubMed]
- Polistina FA, Guglielmi R, Baiocchi C, et al. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiotherapy and Oncology 2011;99:120-3. [PubMed]
- Barney BM, Olivier KR, Miller RC, et al. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol 2012;7:67. [PubMed]
- Singhal D, van Gulik TM, Gouma DJ. Palliative management of hilar cholangiocarcinoma. Surg Oncol 2005;14:59-74. [PubMed]
- Classen M, Hagenmuller F. Endoscopic biliary drainage. Scand J Gastroenterol Suppl 1984;102:76-83. [PubMed]
- Park YJ, Kang DH. Endoscopic drainage in patients with inoperable hilar cholangiocarcinoma. Korean J Intern Med 2013;28:8-18. [PubMed]
- Robson PC, Heffernan N, Gonen M, et al. Prospective study of outcomes after percutaneous biliary drainage for malignant biliary obstruction. Ann Surg Oncol 2010;17:2303-11. [PubMed]
- Saad WE. Transhepatic techniques for accessing the biliary tract. Tech Vasc Interv Radiol 2008;11:21-42. [PubMed]
- Saad WE, Wallace MJ, Wojak JC, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol 2010;21:789-95. [PubMed]
- Rumalla A, Baron TH. Evaluation and endoscopic palliation of cholangiocarcinoma. Management of cholangiocarcinoma. Dig Dis 1999;17:194-200. [PubMed]
- Becker CD, Glattli A, Maibach R, et al. Percutaneous palliation of malignant obstructive jaundice with the Wallstent endoprosthesis: follow-up and reintervention in patients with hilar and non-hilar obstruction. J Vasc Interv Radiol 1993;4:597-604. [PubMed]
- Stoker J, Lameris JS. Complications of percutaneously inserted biliary Wallstents. J Vasc Interv Radiol 1993;4:767-72. [PubMed]
- Davids PH, Groen AK, Rauws EA, et al. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet 1992;340:1488-92. [PubMed]
- Levy MJ, Baron TH, Gostout CJ, et al. Palliation of malignant extrahepatic biliary obstruction with plastic versus expandable metal stents: An evidence-based approach. Clin Gastroenterol Hepatol 2004;2:273-85. [PubMed]
- Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc 2006;63:986-95. [PubMed]
- Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc 2003;57:178-82. [PubMed]
- Prat F, Chapat O, Ducot B, et al. Predictive factors for survival of patients with inoperable malignant distal biliary strictures: a practical management guideline. Gut 1998;42:76-80. [PubMed]
- Abu-Hamda EM, Baron TH. Endoscopic management of cholangiocarcinoma. Semin Liver Dis 2004;24:165-75. [PubMed]
- De Palma GD, Galloro G, Siciliano S, et al. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc 2001;53:547-53. [PubMed]
- Raju RP, Jaganmohan SR, Ross WA, et al. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig Dis Sci 2011;56:1557-64. [PubMed]
- Singhal D, van Gulik TM, Gouma DJ. Palliative management of hilar cholangiocarcinoma. Surg Oncol 2005;14:59-74. [PubMed]
- Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol 2000;19:156-76. [PubMed]
- Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc 2009;69:55-62. [PubMed]
- Molt P, Hopfan S, Watson RC, et al. Intraluminal radiation therapy in the management of malignant biliary obstruction. Cancer 1986;57:536-44. [PubMed]
- Whittington R, Neuberg D, Tester WJ, et al. Protracted intravenous fluorouracil infusion with radiation therapy in the management of localized pancreaticobiliary carcinoma: a phase I Eastern Cooperative Oncology Group Trial. J Clin Oncol 1995;13:227-32. [PubMed]
- González González D, Gouma DJ, Rauws EA, et al. Role of radiotherapy, in particular intraluminal brachytherapy, in the treatment of proximal bile duct carcinoma. Ann Oncol 1999;10 Suppl 4:215-20. [PubMed]
- Milella M, Salvetti M, Cerrotta A, et al. Interventional radiology and radiotherapy for inoperable cholangiocarcinoma of the extrahepatic bile ducts. Tumori 1998;84:467-71. [PubMed]
- Morganti AG, Trodella L, Valentini V, et al. Combined modality treatment in unresectable extrahepatic biliary carcinoma. Int J Radiat Oncol Biol Phys 2000;46:913-9. [PubMed]
- Leung J, Guiney M, Das R. Intraluminal brachytherapy in bile duct carcinomas. Aust N Z J Surg 1996;66:74-7. [PubMed]
- Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology 2003;125:1355-63. [PubMed]
- Richter JA, Kahaleh M. Photodynamic therapy: Palliation and endoscopic technique in cholangiocarcinoma. World J Gastrointest Endosc 2010;2:357-61. [PubMed]
- Alis H, Sengoz C, Gonenc M, et al. Endobiliary radiofrequency ablation for malignant biliary obstruction. Hepatobiliary Pancreat Dis Int 2013;12:423-7. [PubMed]
- Steel AW, Postgate AJ, Khorsandi S, et al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc 2011;73:149-53. [PubMed]


