External beam radiotherapy and bone metastases
Introduction
Bone is a common site for the development of metastatic disease from solid tumors. The most common primary tumor sites that spread to bone are breast, prostate, lung, thyroid, kidney and bone marrow (multiple myeloma), though other primary tumor sites can give rise to osseous metastasis as well. Optimal management requires a collaborative approach, often with input from physicians of various disciplines. Both symptomatic and asymptomatic bone lesions can be palliated with radiation (1). Symptoms of bone metastasis can occur early in a patient’s metastatic course and include localized pain and/or pathologic fracture, functional deficits due to compression of peripheral nerves, nerve roots, or the spinal cord (2,3). Osseous metastasis cause pain directly, through local invasion, and indirectly through alteration of the remodeling activity of osteoblasts and osteoclasts. Pain can be caused by the release of chemical mediators, increased pressure due to tumor, microfractures and stretching of the periosteum (4). Bone metastasis can weaken the bone such that even minor activity such as coughing or sneezing can result in a pathologic fracture. Vertebral bodies can become so compromised that they lose height, which can cause muscle spasms as the muscles struggle to maintain spinal integrity. Nerve involvement can present as radicular or referred pain. Patients perceive pain in various terms, including “burning”, “sharp”, “aching”, and “cramping”. Systemic manifestations of osseous disease include hypercalcemia, fatigue, and anorexia. Treatment of asymptomatic metastasis can prevent pathologic fracture or neurologic compromise from impending spinal cord compression. Asymptomatic lesions of the acetabulum are frequently the targets of radiation therapy as surgical options for acetabular fractures are limited and may result in the patient being unable to bear weight for the remainder of his lifetime.
Radiotherapy for pain
Between half and three-quarters of radiographically apparent osseous metastasis cause discomfort at sometime during a patient’s life. External beam radiation therapy (EBRT) for bone metastasis is one of the most common uses of palliative radiation therapy. EBRT provides effective and time-efficient pain control with few side effects. It can be used to avoid systemic side effects of opioid narcotics. Between 60-80% of patients respond to EBRT and 25-30% of patients have a complete response to treatment. Even radioresistant tumors, such as those due to metastatic sarcoma or renal cell carcinoma, can be well palliated by EBRT. Fractionation schemes with higher dose per fraction may be better in these circumstances (5).
In the vast majority of patients, pain relief does not take effect immediately after the initiation of EBRT. The full palliative effect occurs 4-6 weeks after the completion of treatment; thus, patients must have adequate pain medication regimens so that they can remain still during the 15-20 minutes treatment and comfortable until pain relief occurs. In the Dutch Bone Metastasis Study, the mean time to the onset of pain relief in both arms was three weeks (6). A helpful guide to pain medication dosing has been described by the World Health Organization (7). These regimens may include non-steroidal anti-inflammatory agents, narcotic analgesics, or adjuvant pain medicines such as corticosteroids, nerve-stabilizing medicines, or anti-depressants.
The pain relief provided by EBRT is variable, but typically lasts several months. In a subgroup analysis of patients surviving more than 1 year in the Dutch Bone Metastasis Study, the mean duration of the response, regardless of the fractionation scheme was 29-30 weeks (8). Progression of pain occurred in 55% of survivors at a mean interval of 16-17 weeks. In that scenario, retreatment can be considered.
Radiation therapy works by the creation of double-stranded DNA breaks caused by the interaction between photons, DNA and other molecules. Normal cells more easily repair the damage caused by ionizing radiation, whereas cancer cells may lose the ability to replicate. Tumor death may be preceded by pain relief from EBRT, which suggests a more complicated interplay of cytokines and other cellular mediators on pain receptors.
Impending or pathological fracture
The evaluation of a patient with bone metastasis should include an evaluation for pathologic fracture, especially for those metastasis occurring in weight bearing bones. The morbidity and mortality from a completed fracture are greater than that of a properly managed impending fracture; however, the true risk of pathologic fracture can be difficult to determine (9,10). Accurate prediction of pathologic fractures in various clinical situations remains an active area of investigation. Those bones that bear weight and experience torsional forces are at the highest risk, though any bone sufficiently weakened by tumor may fracture with the slightest force. Surgical stabilization or vertebroplasty, in well-selected patients, followed by EBRT at doses between 20-30 Gy can help the healing process and decrease the incidence of persistent pain due to residual tumor cells (11,12).
There are criteria for determining the risk of pathologic fracture [Harrington’s (13) and Mirels’(14)] and spinal instability (15,16) in the setting of osseous metastasis. Harrington lists four criteria that increase the risk of pathologic fracture: destruction of the metaphysis (>50-75%) or diaphysis (>50% or 2.5 cm), destruction of the subtrochanteric femoral region and persistent pain following radiation therapy (13). Mirels’ criteria assign a score to each of four factors: site, type and size of the lesion and the type of pain. A score of >8 suggests prophylactic fixation should be considered (14). The Spinal Instability Neoplastic Score (SINS) also assigns a score for the location of the lesion, type of pain, spinal alignment, extent of vertebral body collapse and presence of involvement of the posterolateral spinal elements. The composite score then places patients into groups of spinal stability. Patients with a score of 7 or greater should undergo evaluation for prophylactic stabilization (15).
Asymptomatic bone lesions that are at risk for the development of a fracture can be treated with EBRT. This approach decreases the tumor burden and promotes regrowth of normal bone. Bisphosphonates and radiopharmaceuticals also play a role in the prevention of pathologic fracture. These modalities are complementary and are often used in combination.
One reason commonly cited in favor of multi-fraction regimens over those with higher dose per fraction regimens is the potential for pathologic fracture. In the analysis of the RTOG 97-14, there was no difference in the long-term risk of pathologic fracture with the single fraction regimen of 8 Gy when compared to multi-fraction regimen of 30 Gy in 10 fractions (17).
Dose fractionation
Over 100 different fractionation regimens to treat metastatic bone pain are in use worldwide (18). Few areas of radiation are as well studied as the efficacy of single vs. multiple fractionation regimens. Multiple (>30) prospective, randomized trials have been completed during the past three decades; 30 Gy in 10 fractions, 24 Gy in 6 fractions, 20 Gy in 5 fractions, and a single 8 Gy fraction are equivalent in terms of short-term pain relief, mean time to response, and mean duration of response. Table 1 compares the three largest randomized fractionation trials.

Full table
Single fraction treatment provides several advantages including greater patient and caregiver convenience as well as fewer short-term side effects (17). Some physicians reserve this approach for patients with a short life expectancy; however, an unplanned subgroup analysis of patients surviving >52 weeks in the Dutch Bone Metastasis trial suggests that the higher total doses of multi-fraction regimens offer no additional benefit over a single fraction treatment (8,21). In that study and in RTOG 97-14, physicians routinely overestimated patient survival (8,22) In theory, another advantage of the higher dose per fraction approach is the increased the number of double stranded DNA breaks when compared to lower dose per fraction. There is also the potential to overcome the relative radioresistance of certain tumor types, e.g., renal cell carcinoma. There is limited data in this setting as the majority of patients enrolled in these trials had breast, lung or prostate primary tumors.
There are certain circumstances when a higher total dose of 20-30 Gy could be considered. These include bone metastases with a large extraosseous component, osteolytic lesions with impending pathologic fracture in those who are medically inoperable (23) and in those patients with a symptomatic pathologic fracture. In the first two situations, the goal of the longer course in these circumstances is to maximize tumor control and remineralization, issues that are more relevant for those who will likely survive for several months. In the latter scenario, it may be difficult to determine the efficacy of treatment that is complicated by a painful fracture. The proper fractionation in these clinical scenarios remains controversial; a single trial of patients with neuropathic pain from bone metastases did not show superiority for either 20 Gy in 5 fractions or a single 8 Gy fraction (24).
Nearly every series has demonstrated that single fraction courses are more commonly associated with re-treatment to the same painful site than fractionated courses, with rates of 20% versus 8%, respectively. Many attribute this to reluctance on the part of radiation oncologists to give additional fractionated radiation after a fractionated course and the perceived safety of additional treatment after an initial course of a single fraction. Re-treatment increases the response rate by approximately 10%. In addition, there has been less reported benefit to retreatment after multi-fraction regimens than after single fraction regimens (6).
Recently, the NCIC completed a randomized trial of re-treatment. In this study, the initial course of RT varied and included single-fraction and multi-fraction regimens with daily fractions ranging from 3-8 Gy. Patients were stratified by their prior radiation regimen (25). Patients with persistent pain at four weeks were randomized to 8 Gy in a single fraction versus 20 Gy in 5 fractions. At two months, there was no difference in the overall pain response to treatment, pathologic fracture or development of spinal cord or cauda equina compression. Acute toxicity was lower in terms of anorexia and diarrhea in the single fraction arm.
There was a higher incidence of pathologic fracture in weight bearing bones seen in the Dutch Bone Metastasis Study (23) but when corrected for the presence of >30% cortical destruction, the fractionation scheme was no longer statistically significant. In RTOG 97-14, the rates of pathologic fracture in the treated bone (4-5%) and adjacent bone (3-4%) were equivalent (17).
Process of radiation therapy planning and delivery
Patients with documented bone metastasis are referred to radiation oncologists. At the time of the consult, the radiation oncologist reviews all of the relevant clinical data, radiographic studies and performs a history and physical exam. Communication with the other oncologic providers follows that evaluation, as do orders for any additional diagnostic testing or procedures. For patients with a life expectancy of six months or less, consideration should be given to palliative care consultation. When a radiation oncologist determines that EBRT is the most appropriate treatment for a patient, a simulation or radiation planning session is scheduled. The goals of this simulation are to position the patient in a comfortable and reproducible position and obtain a CT scan of the affected area that includes all of the Organs at Risk (OAR) for dose calculations. An alternative to CT simulation is a fluoroscopic simulation. The position also serves to minimize radiation dose to unaffected body regions, e.g., the arms. The dosimetry, or dose planning, is completed next and involves computerized measurement of the best means by which to deliver dose to the intended target while minimizing treatment to adjacent normal tissues. In cases in which the patient has difficulty with transfers or lives far from a radiation treatment facility, it is most efficient to complete the consultation, simulation, and initiation of single fraction therapy during the same day. Some institutions even set aside resources in “Rapid Response Clinics” to accomplish this one-day palliative therapy approach (26). Once the dosimetric analysis has been completed, the physician and the physicist review the plan to ensure accuracy. Prior to the delivery of the first treatment, a verification simulation is performed. This ensures that what was planned behind the scenes actually conforms to the patient set-up on the treatment table. Portal images and/or CT images are obtained to verify that the set-up is correct for the area being treated. Patients are in the treatment room for 15-20 minutes while the radiation is delivered. Treatment is without immediate side effects, other than the potential for discomfort on the treatment table, in the transfer to the treatment table or in the treatment position.
Side effects of EBRT
The acute side effects of radiation therapy are most often predictable based on the region being treated, mild, and manageable with conservative measures. The main systemic side effect is fatigue, though this is typically less than the fatigue associated with the disease or other treatment modalities. Local side effects include skin irritation and reaction, like a mild sunburn; gastrointestinal complaints, such as nausea or diarrhea. Esophagitis or mucositis can result from radiation to mucosal surfaces adjacent to the bone lesion treated. Side effects occur acutely, sub-acutely and in the long-term and are affected by both the daily dose of radiation and the total dose delivered. Fewer acute side effects have been associated with single fraction palliative radiation when compared to multi-fraction regimens (21,27). A “pain flare” or transient increase in bone pain that occurs around the first few fractions of radiation is caused by tumor cell kill and has been reported in between 20-40% of patients (28). This pain flare can be minimized by the use of non-steroidal or steroidal anti-inflammatory medications. Given the limited life expectancy of most patients with metastatic cancer, the acute side effects are much more clinically relevant than late side effects.
There is controversy about the risk of pathologic fracture in the setting of osseous metastasis. In RTOG 97-14, the risk of pathologic fracture was 4-5% (17,27). In the Dutch Bone Metastasis Study, the risk of pathologic fracture was higher in the single fraction arm and occurred at a median of seven weeks. In the multi-fraction arm, the fractures were seen at a median of 20 weeks. They found that cortical destruction >30% was more predictive of pathologic fracture and once this was controlled for, the fractionation regimen became non-significant (23). Multidisciplinary management of patients with significant cortical destruction is essential, as the risks and morbidity of a prophylactic fixation should be weighed against the risks and morbidity of a pathologic fracture in the patient’s remaining lifespan.
Rarely, there are late effects of radiation therapy. By definition, these occur several months to years following the delivery of radiation and can be more serious than the acute side effects of radiation therapy. Acute side effects depend mostly on the total dose delivered, while late effects depend both on the dose per day and the total dose delivered. In other words, larger daily doses of radiation correlate with a higher risk of long-term side effects, though the risk of serious side effects is still very low. This may be due in part to the fact that patients with bone metastases do not typically live long enough to commonly suffer late side effects. However, with improvements in systemic therapies, some patients with bone metastasis may live longer and thus potentially be at risk for the development of late complications that can be associated with short course, high dose per fraction therapy. This has not been clinically significant given the relatively short survival in metastatic cancer and modest total dose delivered with single fraction regimens. In addition, physicians are notorious for overestimating survival. In the Dutch Bone Metastasis Trial, a separate stratification and randomization was performed for patients who were thought to have a better prognosis. After one year, only 53% of those patients were alive (8). On average, physicians overestimate the survival of patients with metastatic cancer by three months, so decision-making must account for this common bias. Four factors that have been associated with a better prognosis include histology (breast or prostate), absence of visceral metastasis, Karnofsky Performance Status and the Functional Assessment of Cancer Therapy (FACT) (22).
Retreatment with EBRT
Re-irradiation of a previously treated site occurs with some frequency. For patients initially treated with multi-fraction regimens, the re-treatment rates are approximately 8%. In comparison, the re-treatment rate for those whose initial course was a single fraction of 8 Gy, the re-treatment rate is 20% (5). Just over half of patients (55%) experience recurrent pain at a previously irradiated site. One randomized trial suggested a smaller benefit to retreatment after multi-fraction regimens (6). In every trial that has studied re-treatment, it was given at the discretion of the treating physician and not mandated by protocol. Physicians seem more likely to offer re-treatment after an initial single fraction versus a more prolonged radiotherapy course (6). Recent consensus conference groups have therefore begun to better define the criteria by which re-treatment should be considered. Given that pain sometimes recedes slowly following radiotherapy over a period of days to weeks, and occurs at a median of three weeks, the minimum interval before re-treatment should be considered is four weeks (29). There are those who typically wait 6 weeks before any decision with regard to retreatment is considered. There had been little prospective data available detailing the risks and the side effect profile for multiple courses of radiation to the same site, though retrospective studies suggested that re-treatment can be given with relatively safety and a 50-70% chance for pain relief (30). The NCIC’s recent trial of fractionation in the re-treatment of bone metastases provided confirmatory evidence that there was no difference in long-term toxicity between single and fractionated radiotherapy courses. Thus single fraction approaches should be considered for their convenience (25).
Highly conformal therapy
Several emerging technologies, such as intensity modulated radiation therapy (IMRT), stereotactic body radiation therapy (SBRT) and proton beam radiation therapy are capable of producing EBRT that is considered highly conformal (31). The goal of these techniques is to deliver high doses to the target while minimizing damage to adjacent structures. Image guided radiation therapy (IGRT) can help to optimize patient positioning (32). IMRT uses an inverse planning process with dose constraints for Organs at Risk (OAR) in the treated volume. SBRT involves the delivery of large, highly conformal doses with fastidious attention paid to dose planning, patient set-up, and localization. This technique may be especially useful in the re-treatment of an area where the spinal cord has reached tolerance due to the initial definitive course of radiation therapy or a previous palliative course of treatment. Proton beam therapy takes advantage of the physical properties of protons to maximize dose to the intended target (33).
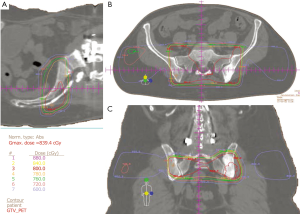
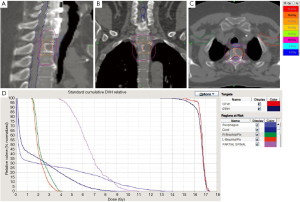
One of the more recent applications of stereotactic radiation is for spine metastases. This approach uses highly conformal radiation to deliver a very high dose of radiation in a small number of fractions. It has been given as part of the initial treatment or in the setting of a previously irradiated area (34). Treatment regimens studied include 30 Gy in 5 fractions, 27 Gy in 3 fractions, 40 Gy in 5 fractions, or 16-24 Gy in a single fraction (35-37). The results of these early trials are promising with prospective, randomized data likely to further define the best use of this technology (38). However, since this is a relatively new approach and there is relatively little data on the long-term effects of very large single doses delivered by these innovative systems, there may be a higher risk of long term side effects than is typically seen with more established treatment approaches due to the higher dose per fraction (39). This is an area of active clinical investigation and the subject of a current RTOG randomized trial (38). Routine use of stereotactic spine RT should not be employed until sufficient evidence from clinical trials justifies the substantive increase in cost when compared to standard EBRT. Figure 1A-C illustrates treatment of a sacroiliac metastasis with 8 Gy in a single fraction in the axial, coronal, and sagittal planes. Figure 2A-D Illustrates SBRT to a vertebral body metastasis with 16 Gy in a single fraction.
Guidelines and quality measures
Multiple randomized trials have compared single-fraction approaches to palliative radiation to multiple different multi-fraction approaches. The overwhelming evidence suggests equivalence in efficacy yet increased cost and inconvenience of multi-fraction approaches, yet there is still a great deal of variability of approaches in use by radiation oncologists. One survey revealed that 101 different dose fractionation schemes were employed worldwide for this single clinical circumstance (18). A recent study in British Columbia demonstrated wide variability within practices and between practices with a range in use of single fraction radiation from 24-72% (16). The American Society for Radiation Oncology (ASTRO) and the American College of Radiology (ACR) have developed guidelines (40-42). Four fractionation schemes are equivalent in the successful management of painful bone metastases: 30 Gy in 10 fractions, 24 Gy in 6 fractions, 20 Gy in 5 fractions, and a single 8 Gy fraction. A trade-off between increased retreatment rates for single fraction radiation and increased convenience exists. Additionally, the guidelines differentiate between treatment approaches that have proven to be effective through clinical trials and those approaches that require further investigation before being used routinely. The use of one of the four approved fractionation schemes is considered a measure of quality as determined by the National Quality Forum (NQF) (43). The NQF is a non-profit organization tasked with assessing healthcare priorities and providing a means to measure and report on the performance of healthcare providers and healthcare facilities. In addition, appropriate fractionation schemes for painful uncomplicated bone metastasis have been incorporated into several specialty groups’ “Top 5” in an initiative called “Choosing Wisely” (44), a program started to help physicians become better financial stewards of healthcare use.
Summary
The treatment of osseous metastasis continues to be a significant clinical problem for patients with cancer. Most commonly, pain at the site of a metastatic deposit triggers a referral to radiation oncology for intervention, thought there is also a role in treatment of asymptomatic lesions at risk for pathologic fracture. EBRT continues to be the primary treatment for painful bone metastases. The treatment of bone metastasis is a multi-disciplinary effort requiring coordination between the radiation oncologist and other specialists including medical oncologists, surgeons, palliative medicine specialists, and physiatrists. Short course treatments effectively palliate pain, with many patients best treated by a single fraction. Short- and long-term toxicity from EBRT is typically minimal, self-limited and managed conservatively. Highly conformal approaches for bone metastases shows great promise, especially in patients with recurrent pain in the spine after previous conventionally fractionated curative therapy and remain an area of active clinical investigation. Bone metastases treatment guidelines and quality measures provide data-derived direction for the management of patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Galasko CS. The anatomy and pathways of bone metastasis. In: Weiss L, Gilbert AH. eds. Bone Metastasis. Boston, MA: GK Hall, 1981:49-63.
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588-94. [PubMed]
- Kamby C, Vejborg I, Daugaard S, et al. Clinical and radiologic characteristics of bone metastases in breast cancer. Cancer 1987;60:2524-31. [PubMed]
- Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain 1997;69:1-18. [PubMed]
- Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007;25:1423-36. [PubMed]
- van der Linden YM, Lok JJ, Steenland E, et al. Single fraction radiotherapy is efficacious: a further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int J Radiat Oncol Biol Phys 2004;59:528-37. [PubMed]
- WHO. World Health Organization Pain Ladder 2010 [cited 2013 9 Feb 3013]. Available online: http://www.who.int/cancer/palliative/painladder/en/
- van der Linden YM, Steenland E, van Houwelingen HC, et al. Patients with a favourable prognosis are equally palliated with single and multiple fraction radiotherapy: Results on survival in the Dutch Bone Metastasis Study. Radiother Oncol 2006;78:245-53. [PubMed]
- Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol 1991;9:509-24. [PubMed]
- Springfield D. Pathologic Fractures. Rockwood and Green’s Fractures in Adults. 5th edition ed. Philadelphia: Lippincott Williams Wilkins, 2001.
- Koswig S, Budach V. Remineralization and pain relief in bone metastases after after different radiotherapy fractions (10 times 3 Gy vs. 1 time 8 Gy). A prospective study. Strahlenther Onkol 1999;175:500-8. [PubMed]
- Townsend PW, Smalley SR, Cozad SC, et al. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys 1995;31:43-9. [PubMed]
- Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect 1986;35:357-81. [PubMed]
- Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 1989;256-64. [PubMed]
- Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol 2011;29:3072-7. [PubMed]
- Olson RA, Tiwana M, Barnes M, et al. Use of Single Fraction Palliative Radiation Therapy for Bone Metastases: Population-Based Practice Patterns in British Columbia Over a 5-Year Period. Int J Radiat Oncol Biol Phys 2013;87:S88-9.
- Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 2005;97:798-804. [PubMed]
- Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys 2009;75:1501-10. [PubMed]
- 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: Randomised comparison with a multifraction schedule over 12 months of patient follow-up. Bone Pain Trial Working Party. Radiother Oncol 1999;52:111-21. [PubMed]
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101-9. [PubMed]
- Foro Arnalot P, Fontanals AV, Galceran JC, et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother Oncol 2008;89:150-5. [PubMed]
- Hartsell WF, Desilvio M, Bruner DW, et al. Can physicians accurately predict survival time in patients with metastatic cancer? Analysis of RTOG 97-14. J Palliat Med 2008;11:723-8. [PubMed]
- Van der Linden YM, Dijkstra PD, Kroon HM, et al. Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg Br 2004;86:566-73. [PubMed]
- Roos DE, Turner SL, O’Brien PC, et al. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05). Radiother Oncol 2005;75:54-63. [PubMed]
- Chow E, van der Linden YM, Roos D, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol 2014;15:164-71. [PubMed]
- Thavarajah N, Wong K, Zhang L, et al. Continued success in providing timely palliative radiation therapy at the Rapid Response Radiotherapy Program: a review of 2008-2012. Curr Oncol 2013;20:e206-11. [PubMed]
- Hartsell WF, Scott C, Bruner DW, et al. Phase III randomized trial of 8 Gy in 1 fraction vs. 30 Gy in 10 fractions for palliation of painful bone metastases: preliminary results of RTOG 97-14. Int J Radiat Oncol Biol Phys 2003;57:S124. [PubMed]
- Loblaw DA, Wu JS, Kirkbride P, et al. Pain flare in patients with bone metastases after palliative radiotherapy--a nested randomized control trial. Support Care Cancer 2007;15:451-5. [PubMed]
- Chow E, Hoskin P, Mitera G, et al. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys 2012;82:1730-7. [PubMed]
- Huisman M, van den Bosch MA, Wijlemans JW, et al. Effectiveness of reirradiation for painful bone metastases: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 2012;84:8-14. [PubMed]
- Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol 2010;7:44-54. [PubMed]
- Jaffray D, Kupelian P, Djemil T, et al. Review of image-guided radiation therapy. Expert Rev Anticancer Ther 2007;7:89-103. [PubMed]
- Allen AM, Pawlicki T, Dong L, et al. An evidence based review of proton beam therapy: the report of ASTRO’s emerging technology committee. Radiother Oncol 2012;103:8-11. [PubMed]
- Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys 2008;71:652-65. [PubMed]
- Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol 2012;13:395-402. [PubMed]
- Garg AK, Shiu AS, Yang J, et al. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer 2012;118:5069-77. [PubMed]
- Jhaveri PM, Teh BS, Paulino AC, et al. A dose-response relationship for time to bone pain resolution after stereotactic body radiotherapy (SBRT) for renal cell carcinoma (RCC) bony metastases. Acta Oncol 2012;51:584-8. [PubMed]
- RTOG. RTOG 0618: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Operable Stage I/II Non-Small Cell Lung Cancer 2006. Available online: http://www.google.com/url?sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=2&ved=0CDYQFjAB&url=http%3A%2F%2Fwww.rtog.org%2FClinicalTrials%2FProtocolTable%2FStudyDetails.aspx%3Faction%3DopenFile%26FileID%3D4650&ei=9b4TUaD7B6yE2QXYooCgCA&usg=AFQjCNH3XRG72GBwcAqbx2WYC37kQV-SoQ&sig2=M25iWW62dHbnx_PJnOTUtQ
- Lo SS, Sahgal A, Chang EL, et al. Serious complications associated with stereotactic ablative radiotherapy and strategies to mitigate the risk. Clin Oncol (R Coll Radiol) 2013;25:378-87. [PubMed]
- Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, et al. ACR Appropriateness Criteria ® spinal bone metastases. J Palliat Med 2013;16:9-19. [PubMed]
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [PubMed]
- Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria(R) non-spine bone metastases. J Palliat Med 2012;15:521-6. [PubMed]
- NQF. #1822 External Beam Radiotherapy for Bone Metastasis. 2012.
- ABIM. Choosing Wisely. 2012.


