Safety evaluation of a potential ablation agent—hydrochloric acid in the rabbits’ model
Core tip
In previous report, it had confirmed that hydrochloric acid (HCl) was an effective ablation agent with single using or combing with radiofrequency ablation. This article systematically evaluated the safety of HCl for the first time. In this study, we found that HCl can cause some changes in arterial blood gas status, hematology and hepatic and renal functions. However, these changes were tolerant and temporary. In imaging and histopathological studies, the lesion caused by HCl was limited. What is more important was that 10% and 20% HCl are more controllable than 30% HCl.
Introduction
Many local ablative therapies greatly improved the prognosis of unresectable or recurrent hepatocellular carcinoma in the last two decades (1-4). These mainly included percutaneous ethanol injection (PEI), percutaneous acetic acid injection (PAI), radiofrequency ablation (RFA), cryoablation, and microwave ablation (5-7). However, the overall survival rate of hepatocellular carcinoma was still unsatisfactory (8-10). Recently, some researchers applied HCl in local ablative therapies. It had satisfactory effect that 1-, 2-, 3-year survival rates reached 100%, 90%, 85%, respectively (11). In experiment studies, HCl also caused more completely necrotic and lager ablation lesion than acetic acid or ethanol. Meanwhile, other researchers found that RFA combined with HCl could significantly enlarge the ablation lesion. They created ablation lesion of 5.51 cm × 4.49 cm in excised porcine livers, which was much larger than combined saline with RFA (12). Therefore, HCl may be a potential ablation agent both for single use and combining with RFA in the future.
The principle of the fact that HCl can destruct tumor is that it has the ability to destroy the integrity of cell membrane and coagulate the protein. It is similar to ethanol and acetic acid, but the effort is much more prominent. Furthermore, HCl is a strong electrolyte solution which may effectively delay the carbonization or desiccation around electrode and slightly increase the tissue boiling point (13-16). This is also the reason why saline, ethanol and acetic acid can effectively increase ablation area. However, it seems that the effect of HCl is much more remarkable, which consists with experiment results.
However, a number of issues still remain. For instance, whether local injection with HCl in the liver affects acid-base balance, hematology and liver and renal functions? Moreover, the controllability of HCl is also unknown. In this study, we evaluate the safety of local injection with HCl in the liver by examining the general conditions, body weight, arterial blood gas status, hematology and hepatic and renal functions of the animals, as well as by performing imaging and histopathological studies.
Materials and methods
Ethics
All animal experiments were performed in accordance with the protocol, approved by institutional animal care and use committee of Sun Yat-Sen University (Permit Numbers: 2012-0404) and in compliance with the Declaration of Helsinki [2000] of the World Medical Association.
Animals and agents
Eighty adult New Zealand rabbits of equal genders were purchased from Medical Animal Center in Guangdong, China. The rabbits weighted between 2.0 and 2.5 kg. All animals were housed individually and handled in accordance with the NIH guidelines for laboratory animal cultivation. HCl was purchased from HuaYi Medicinal Auxiliary Materials Manufacturing Limited Pharmaceutical Company, Chengdu, China. Various HCl concentrations of 10%, 20% and 30% were diluted from the original solution of 38% HCl in aseptic condition. All animals were fasted for 12 hours before the experiment.
Experimental design
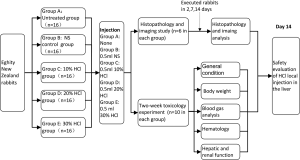
A total of 80 rabbits were divided into five groups of equal size (n=16): the untreated, normal saline (NS) control, 10% HCl, 20% HCl and 30% HCl groups via randomization stratified by body weight and gender. The untreated group was not injected, whereas animals in other groups received an equal volume of 0.5 mL of corresponding perfusate in the liver. Each injection into the rabbit liver was through a 15 cm 21G single-hole spinal needle, which inserted 3 cm into the rabbit liver. And the injection rate was 0.2 mL/min, maintained by a peristaltic pump.
Each group was divided into two subgroups: ten rabbits for the two-week toxicology study and six for the imaging and histopathology study. Body weight and physiologic conditions were monitored during the study. In the toxicology study, under general anesthesia (with 1% pentobarbital, 3 mL/kg), the femoral artery of each rabbit was cannulated and the liver was surgically exposed for blood sample collection and intrahepatic injection, respectively. The blood samples were collected at time points of hours 0, 0.25, 0.5, 1, 3 and 6. The incisions at the abdomen and leg were closed by layered sutures after blood collection and injection. We collected the blood samples from ear artery at time points of days 2, 7 and 14. We collected 0.5 mL of blood sample for blood routine tests, 0.5 mL for arterial blood gas analysis and 1 mL for hepatic and renal function tests. All rabbits were euthanized at day 14. In the imaging and histopathology study, magnetic resonance imaging (MRI) scan was first performed and two rabbits from each group were killed at each time points of days 2, 7 and 14 to obtain the liver specimens (Figure 1).
Blood examinations
Arterial blood gas analysis was performed on State Profile CCX (NOVA Company, USA). The blood samples were drawn with a 1-mL glass syringe that was coated with heparin calcium. The samples were tested for 15 minutes for measuring Na+, K+, Ca2+, lactic acid, PH, actual base excess and bicarbonate radical content.
Hematologic analysis was performed on XE-5000 (Sysmex, Japan). The blood samples were drawn with a 2-mL glass tube that was coated with ethylene diamine tetraacetic acid (EDTA). The samples were kept at room temperature to measure the white blood cell count, red blood cell count, hemoglobin concentration and platelet count.
Hepatic and renal function tests were performed on Labospect 008 (HITACHI, Japan) by using a biochemistry kit (Wako, Japan). The samples were drawn with a 4-mL glass tube and centrifuged at 3,500 ×g for 5 min. The serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total protein, albumin, A/G, urea nitrogen (BUN), creatinine (CRE) and C-reactive protein (CRP) were measured.
Histopathologic examination
Hepatic tissues were preserved in 10% neutral-buffered formalin, processed and trimmed, embedded in paraffin, sectioned to a thickness of 4 µm, and stained with hematoxylin and eosin. All tissues were observed for pathologic changes by using Nikon Eeclipse-80i (Japan). Gross and microscopic data were entered into a pathology software program NIS-Elements. According to the toxicological pathology criteria of Shackelford et al. (17), the five grades used to evaluate the extent of lesions in the tissue are as follows: no lesion (grade 0), minimal (grade 1), mild (grade 2), moderate (grade 3) and marked (grade 4).
Imaging study
Imaging study was performed with a 1.5 T MRI system with a nuclear spin of 288 Hz (Signa HDx; GE Healthcare, USA, fairfield.). The thickness of each layer was 4 mm and the gap was 0 mm. All MRI images in this study were T2-wighted images (T2WI) with TR/TE of 5000/85.
Statistical methods
The data were reported as mean ± standard error (SE). The body weight, water and food consumptions, arterial blood gas analysis, hematology results, and hepatic and renal functions were first compared among the untreated, NS control, 10% HCl, 20% HCl and 30% HCl groups with repetitive measurement deviation analysis at an alpha of 0.05. Multiple comparison tests with Bonfferoni were performed when the difference on the group or time factor was statistically different at an alpha of 0.005. The results of the imaging study and histopathological observations were compared using multiple comparisons with Bonfferoni at an alpha of 0.0167. This procedure was performed using commercially available software (Version 16.0, SPSS Inc., Chicago, USA).
Results
General conditions
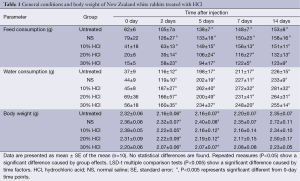
The animal models we tolerated the intrahepatic injection of HCl without observable toxicity. The wounds healed well in all surviving rabbits who recovered to an active status in day 5. No significant differences were observed between the untreated and HCl-treated groups during the experiment. The interaction between treatment effects and time effects was also not significant. Food and drinking water consumptions were markedly reduced in all groups during the first two postoperative days (P<0.0001). Compared with the time point on day 14, the food consumption decreased by 59%, 50%, 73%, 85% and 88% in the untreated, NS, 10% HCl, 20% HCl and 30% HCl groups, respectively, and water consumption decreased by 84%, 81%, 84%, 74% and 78%, respectively, in the first two days, as shown in Table 1.
Full table
Body weight
No significant weight differences were observed between the untreated and HCl-treated groups during the experiment. The interaction between treatment effects and time effects was also not significant. The weight of all animals gradually decreased by 7%, 2%, 7%, 4%, 6% in the untreated, NS, 10% HCl, 20% HCl and 30% HCl groups, respectively, before day 5 and then gradually increased (P<0.0001), as shown in Table 1.
Arterial blood gas analysis
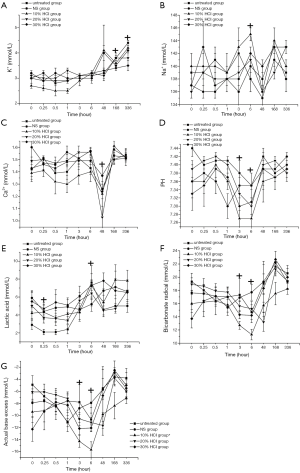
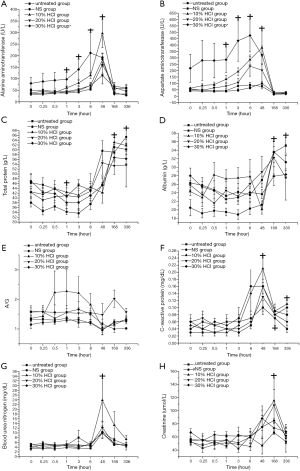
All items had no significant differences in interaction between treatment effects and time effects and had no significant differences in electrolyte, pH and bicarbonate radical levels between the untreated and HCl-treated groups during the experiment. K+ content was stable at a low level from hours 0 to 6. A significant increase was observed on day 7 and lasted until day 14 (P<0.0001, multiple comparison tests) as shown in Figure 2A. Na+ content decreased on day 2, and then recovered to the level of hour 0 on day 7 (P<0.0001, multiple comparison tests, Figure 2B). Ca2+ content was stable from hour 0 to 6, but markedly decreased on day 2 and gradually recovered from day 7 to day 14 (P<0.0001, multiple comparison tests, Figure 2C). PH value gradually decreased during the first six hours, and the minimum value (pH=7.27) was observed at hour 6. On day 2, the pH value recovered to the initial level (P=0.004, multiple comparison tests, Figure 2D). A significant difference in lactic acid content was observed between the untreated and HCl-treated groups (P<0.0001, repeated measures AVNOA). The lactic acid content in the 30% HCl group was higher than in the untreated group. The lactic acid content slightly decreased at hour 0.25 and then gradually increased until the maximum value at hour 6. On day 2, the lactic acid content returned to the initial level (P=0.002, multiple comparison tests, Figure 2E). Bicarbonate radical content and actual base excess gradually decreased during the first 6 hours and then recovered from day 2 to day 14 (P=0.001, multiple comparison tests, Figure 2F,G). The actual base excess in the 10% HCl group was lower than in the untreated group (P=0.003, repeated measures AVNOA).
Hematology results
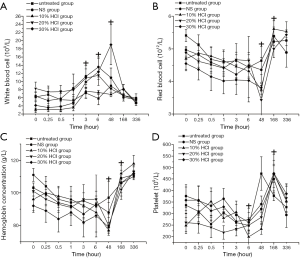
There were no significant differences in interaction between treatment effects and time effects and hematology between the untreated and HCl-treated groups during the experiment. Before day 2, the white blood cell count gradually increased at first but gradually decreased to the initial level on day 7. Significant differences were observed from hour 3 to day 2 (P<0.0001, multiple comparison tests, Figure 3A). Before day 2, the red blood cell count and hemoglobin concentration continued to decrease markedly and then recovered to the initial level of hour 0 on day 14 (P=0.002, multiple comparison tests, Figure 3B,C). Before hour 6, the platelet count continued to decrease markedly then rapidly increased on day 2 until the maximum value on day 7. After this time point, the platelet count returned to the initial level on day 14 (P=0.003, multiple comparison tests, Figure 3D).
Hepatic and renal function
There were no significant differences in interaction between treatment effects and time effects. ALT, AST, and albumin levels were markedly different between the untreated and HCl-treated groups (P=0.126), whereas all other items had no significant differences. ALT and AST levels began to increase markedly at hour 1 until the maximum value on day 2. On day 7, the ALT and AST levels of all groups decreased to that of hour 0 (P<0.0001, multiple comparison tests, Figure 4A,B). Further analysis showed that in the 30% HCl group, ALT and AST levels were markedly higher in comparison to other groups (P=0.002, multiple comparison tests). Total protein content was kept at a low level during the first 6 hours and then gradually increased from day 2 to day 14 (P<0.0001, multiple comparison tests, Figure 4C). Albumin content was lowered before day 2 and then rapidly increased on day 7 (P<0.0001, multiple comparison tests, Figure 4D). A/G had no obvious change along during the experiment (Figure 4E). CRP markedly increased at hour 6 until reaching the maximum value on day 2 and the value gradually decreased up to day 14 (P<0.0001, multiple comparison tests, Figure 4F). BUN and CRE levels began to increase at hour 6 until reaching the maximum value on day 2 and day 7, respectively. On day 14, the values were all normalized (P<0.0001, multiple comparison tests, Figure 4G,H).
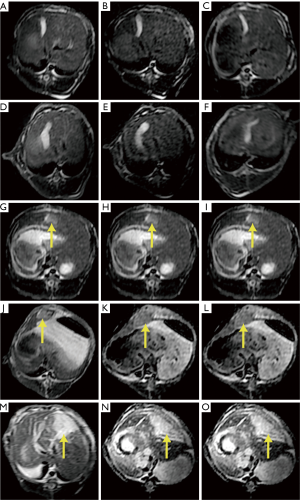
Imaging study
In the untreated and NS groups, no obvious lesions were observed on T2WI at all three time points (Figure 5A-F). In the HCl-treated groups, the lesions were homogenously hyperintense on T2WI, and a clear-cut borderline was observed between the normal tissue and the lesion. The sizes of the lesion were (16±2) mm × (18±2) mm, (18±2) mm × (19±1) mm, and (19±1) mm × (23±1) mm in the 10%, 20% and 30% HCl groups, respectively. The lesions in the 30% HCl group were markedly larger than that in the 10% or 20% HCl groups (P<0.0001, multiple comparison tests, Figure 5G-O, Table 2). All lesions had no significant changes in signal intensity and dimension at all three time points.

Full table
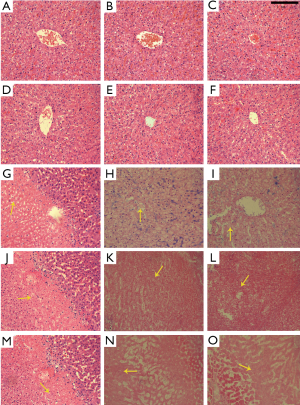
Histopathological observations
No significant pathological changes were observed in the untreated and NS control groups (Figure 6, Figure 6A-F) contrary to the HCl-treated groups. On day 2, the pathological changes involved the degeneration of shrunken hepatocytes with pyknotic nuclei with many infiltrating inflammatory cells (Figure 6G,J,M). On days 7 and 14, the cellular structure was completely lost, and only a small amount of the residual nuclear structure was observed at a high magnification (Figure 6H,I,K,L,N,O). In addition, the 30% HCl group had a more serious necrosis than the low concentration HCl groups based on pathologic study results (Figure 6O).
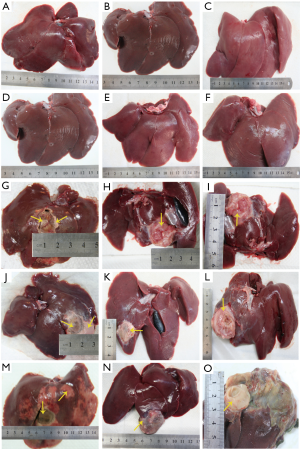
In the untreated and NS control groups, no obvious liver damage was observed in the rabbits during the experiment (Figure 7, Figure 7A-F). However, in the HCl-treated groups, liver damage was obvious in all rabbits. At day 2, coagulated necrosis was observed in the area of the local injection with HCl in the liver, and no evident adhesion to the adjacent organs was observed. The sizes of the completely coagulated necrosis were (19±1) mm × (20±1) mm, (20±1) mm × (21±1) mm, and (19±1) mm × (22±2) mm in the 10%, 20% and 30% HCl groups, respectively. Some minor damaged areas (the damage was only limited on the surface) were also observed around the completely coagulated necrosis. The minor damaged area in the 30% HCl group was larger than in the other HCl groups (Figure 7G,J,M). At days 7 and 14, the damaged area was surrounded by a layer of fiber tissue (Figure 7H,I,K,L,N,O). The damaged area seriously adhered to the surrounding tissue and causing the separation of them became very difficult .The sizes of the coagulated necrosis were (22±3) mm × (24±1) mm, (23±2) mm × (25±1) mm and (22±2) mm × (25±3) mm in the 10%, 20%, 30% HCl groups, respectively. Moreover, based on histological observations, the adjacent organs had no obvious damage, and all the damaged area were limited in the liver.
Discussion
The physical and chemical properties of HCl include low impedance, strong electric conductivity, high boiling point and high specific heat capacity (18,19). These properties make HCl a favorable ablation agent and this finding has been confirmed by our previous experiment (12). Moreover, HCl is an endogenous substance and the main component in human gastric juice. HCl has been recorded in Chinese pharmacopoeia to be capable of treating achlorhydria (20). However, HCl has cytotoxic effects on biological tissues and can induce water, electrolyte and acid-base balance disorders when used improperly on patients. The risks of HCl solution administration depend on its concentration. Base on the classification of HCl in the European Union, concentrations between 10% and 25% can cause irritation whereas concentrations above 25% can cause corrosion (21). Therefore, we study the safety of local injection of various concentrations of HCl here.
The changes in the general conditions and body weight of the animal model are temporary and recoverable during the experiments. These changes are likely due to anesthesia and surgical interventions and may be caused by gastrointestinal dysfunction (22,23). Additionally, the increase energy and protein consumptions may also contribute to these phenomena. All of these changes disappeared on day 7.
Arterial blood gas analysis indicates many significant statistical changes during the experiment. Preoperative fasting and blood loss are the important factors for the changes in K+, Na+, especially for Ca2+ which has a dramatically change at 48 hours. These two factors increase electrolyte loss and decrease electrolyte uptake. Indeed, the majority of the electrolytes decrease at the beginning of the experiment. The main reasons behind the changes in pH and other buffer substances are anesthetic effect and blood loss. Pentobarbital has a strong inhibition effect on the central nervous system and also restrains breathing frequency and amplitude (24-26). Respiratory depression causes body hypoxia and enhances anaerobic respiration. Blood loss causes histanoxia and induces metabolic acidosis. In combination, all these effects eventually lead to a decrease in pH and other changes in the buffer substance, which is well tolerated, recoverable and not life-threatening.
At the start of the experiment, statistically significant changes are observed in the hematology results. Operation injuries and inflammatory responses could induce a dramatic increase in white blood count because the resistance to harmful stimuli increases. Blood loss is the main reason for the decrease in red blood count, hemoglobin concentration, and platelet count at the start of the experiment. Based on experimental data, these hematology changes are temporary and restorable.
Hepatic and renal functions show statistically significant changes during the experiment. The increase in ALT and AST levels is higher in the 30% HCl group than in the other groups because of the more severe liver damage in the 30% HCl group relative to the other groups. The decrease in the total protein and albumin contents at the beginning of the experiment may be related to preoperative fasting. Liver damage and absorption of necrotic tissue are the main reasons for the significant increase in BUN and CRE levels during the experiment. These phenomena are very common with radiofrequency (27). The other interventional therapies such as cryoablation and microwave ablation also easily result in obvious increase in ALT and AST levels after therapies are performed (28). CRP level is higher before day 7, which reflects the degree of inflammatory reaction after surgery. All of these changes recover at the end of the experiment.
The imaging and histological results indicate that local injection with HCl induces limited liver damage, and the MRI results show that the induce lesion in the 30% HCl group is larger than that in the other groups. However, no significant differences are observed between the 30% HCl and the other groups in the histology study. The histology results indicate that this phenomenon may be due to the minor damaged area. We infer that these minor damaged areas have a close relationship to the volatility of HCl. This inference is in accordance with the experiment results where the 30% HCl group have the strongest volatility and the minor damaged area in the HCl-treated groups is larger based on histology and imaging study results. Moreover, according to Shackelford et al. (17), the local injection of HCl causes local and marked (grade 4) tissue lesion. Therefore HCl induces complete necrosis, which is in accordance with the report of Weijian et al. (11). A positive correlation is observed between the degree of necrosis and the concentration of HCl. This phenomenon shows that HCl has a strong tissue destruction effect.
This study has several limitations. Firstly, the experiment is performed on normal liver tissues rather than on tumor tissues of rabbits. The tumor tissue may have properties that are different from the normal tissue. This difference may influence the diffusion of HCl. Secondly, this study is only performed with HCl but not in combination with RFA. Besides, Hsieh CH et al. reported a clinical case about a serious arterial occlusion that was induced by the injection of HCl in the artery (29). Therefore, the impact of HCl on the vascular tissue should be further studied, particularly when the tumor is positioned adjacent to the major blood vessels.
In summary, HCl appears to be an effective ablation agent or may be potential to increase ablation area if combined with RFA, which does not cause serious side effects when locally injected in the liver. Additionally, HCl at a low concentration is more controllable than at a high concentration.
Acknowledgements
The authors gratefully acknowledge, Xiongying Jiang and Yanyang Zhang for data collection in this study. We also thank to Xiao Jiang and Wei Liu for the editing the manuscript.
Authors’ contributions: Wang Y designed the research, performed the majority of experiments and wrote the manuscript; Gao F and Gu YK also performed the majority of experiments; Liu WL and Wang J contributed new reagents and analytic tool; Huang JH provided the collection of all the material in addition to providing financial support for this work.
Funding: Natural Science Foundation of China, No.81371652 and Guangdong Provincial Science & Technology Projection, No.2012B031800120.
Disclosure: The authors declare no conflict of interest.
References
- Bowles BJ, Machi J, Limm WM, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg 2001;136:864-9. [PubMed]
- Kettenbach J, Blum M, Kilanowicz E, et al. Percutaneous radiofrequency ablation of liver cell carcinoma: a current overview. Radiologe 2004;44:330-8. [PubMed]
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012;262:43-58. [PubMed]
- Tranberg KG. Percutaneous ablation of liver tumours. Best Pract Res Clin Gastroenterol 2004;18:125-45. [PubMed]
- Beaugrand M, N’kontchou G, Seror O, et al. Local/regional and systemic treatments of hepatocellular carcinoma. Semin Liver Dis 2005;25:201-11. [PubMed]
- Hinshaw JL, Lee FT Jr. Cryoablation for liver cancer. Tech Vasc Interv Radiol 2007;10:47-57. [PubMed]
- Lencioni R, Crocetti L. Image-guided ablation for hepatocellular carcinoma. Recent Results Cancer Res 2013;190:181-94. [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [PubMed]
- Gluer AM, Cocco N, Laurence JM, et al. Systematic review of actual 10-year survival following resection for hepatocellular carcinoma. HPB (Oxford) 2012;14:285-90. [PubMed]
- Maggs JR, Suddle AR, Aluvihare V, et al. Systematic review: the role of liver transplantation in the management of hepatocellular carcinoma. Aliment Pharmacol Ther 2012;35:1113-34. [PubMed]
- Weijian F, Zan L, Suhong H, et al. Destructive effect of percutaneous hydrochloric acid injection therapy for liver cancer--a preliminary experimental and clinical study. Gan To Kagaku Ryoho 2006;33:1852-6. [PubMed]
- Luo RG, Fao F, Huang JH, et al. Diluted hydrochloric acid generates larger radiofrequency ablation lesions in excised porcine livers. Diagn Interv Radiol 2013;19:145-9. [PubMed]
- Giorgio A, Tarantino L, de Stefano G, et al. Percutaneous sonographically guided saline-enhanced radiofrequency ablation of hepatocellular carcinoma. AJR Am J Roentgenol 2003;181:479-84. [PubMed]
- Goldberg SN, Gazelle GS, Dawson SL, et al. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol 1995;2:399-404. [PubMed]
- Goldberg SN, Gazelle GS, Halpern EF, et al. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol 1996;3:212-8. [PubMed]
- Kettenbach J, Köstler W, Rücklinger E, et al. Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients. AJR Am J Roentgenol 2003;180:1537-45. [PubMed]
- Shackelford C, Long G, Wolf J, et al. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol 2002;30:93-6. [PubMed]
- Lide DR. eds. CRC Handbook of Chemistry and Physics, 81st Edition, Gaithersburg, CRC press, 2000.
- Robert P. eds. Perry’s chemical engineers’ handbook, 6th edition, New York, McGraw Hill, 1997.
- Commission Cp. The second of 2010 version of the People’s Republic of China pharmacopoeia. Beijing: China Medical Science Press, 2010:1215. Available online: http://www.chp.org.cn/cms/home/
- EUR-lex. Council Directive 67/548/EEC of 27 June 1967 on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances,retrieved 2., 2008:1103. Available online: http://eur-lex.europa.eu/LexUriServ/
- Hahnenkamp K, Herroeder S, Hollmann MW. Regional anaesthesia, local anaesthetics and the surgical stress response. Best Pract Res Clin Anaesthesiol 2004;18:509-27. [PubMed]
- Somri M, Matter I, Parisinos CA, et al. The effect of combined spinal-epidural anesthesia versus general anesthesia on the recovery time of intestinal function in young infants undergoing intestinal surgery: a randomized, prospective, controlled trial. J Clin Anesth 2012;24:439-45. [PubMed]
- Hurlé MA, Dierssen MM, Flórez J. Mechanism of the respiratory action of pentobarbital at the medullary and pontine levels. Eur J Pharmacol 1986;125:225-32. [PubMed]
- Murray A, Bellville JW, Comer W, et al. Respiratory effects of quazepam and pentobarbital. J Clin Pharmacol 1987;27:310-3. [PubMed]
- Wixson SK, White WJ, Hughes HC Jr, et al. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on arterial blood pH, blood gases, mean arterial blood pressure and heart rate in adult male rats. Lab Anim Sci 1987;37:736-42. [PubMed]
- Koda M, Murawaki Y, Hirooka Y, et al. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: An analysis of 16,346 treated nodules in 13,283 patients. Hepatol Res 2012;42:1058-64. [PubMed]
- Bertot LC, Sato M, Tateishi R, et al. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol 2011;21:2584-96. [PubMed]
- Hsieh CH, Lin GT. Corrosive injury from arterial injection of hydrochloric acid. Am J Emerg Med 2005;23:394-6. [PubMed]