Palliative radiotherapy for advanced malignancies in a changing oncologic landscape: guiding principles and practice implementation
Introduction
Palliative radiotherapy has provided a cornerstone of symptom management for patients with advanced and metastatic cancer for more than 100 years (1). Since shortly after the discovery of the X-ray in 1896, clinicians have been utilizing radiotherapy to help patients to manage bothersome symptoms of advanced cancer, including pain and bleeding. The ability to palliate symptoms of advanced cancer that were deeper than skin or bone improved dramatically with the advent of megavoltage radiation in the 1950s and 1960s, allowing radiation therapy to penetrate deeper and provide relief from symptoms not previously well palliated with kilovoltage irradiation, including neurologic symptoms from brain metastases (2) and obstructive symptoms from visceral tumors. By the 1960s, clinicians had outlined certain principles of palliative radiotherapy that were distinct from the principles of “curative” or “definitive” radiotherapy: namely, that the provision of radiotherapy delivered for its palliative effect mandated minimization of side effects, consideration of patient convenience and consideration of cost (3). In addition, there was increasing recognition that radiation oncologists must provide palliation in the context of therapies provided by other clinicians, including chemotherapy, surgery, pharmacologic symptom management, and anesthesiology and other interventional procedures.
Over the past 50 years, there have been many advances in cancer imaging, cancer biology, and therapeutic advances (including surgery, radiation techniques and chemotherapy, as well as biologic therapies) that have changed the experience of illness for patients with advanced cancer. Moreover, the growth of the hospice and palliative care movements from the 1960s to the 1980s has allowed for intensive, specialist-level attention to symptom management for patients with advanced cancer (4). The integration of palliative radiotherapy with hospice and palliative care teams has not always been deliberate, as surveys show that only 10% of hospice providers consider radiation oncologists to be part of the palliative care team (5). This is despite up to 40% of patients receiving some form of radiotherapy during their treatment course, according to a SEER-Medicare population-based study of more than 50,000 patients with metastatic lung cancer, prostate cancer, breast cancer and colorectal cancer who were diagnosed between 2000 and 2007 (6).
Palliative radiotherapy remains among the most effective methods for symptomatic control in advanced malignancies, but there are opportunities for improved collaboration among medical oncologists, palliative care clinicians and radiation oncologists in the provision of palliative care for patients with advanced and metastatic cancer (7). In this context, the current review will outline the indications for palliative radiotherapy, the selection of appropriate dose-fractionation schemes, the opportunities for integrating advanced technologies in palliative radiotherapy programs, and the possibilities for implementing collaborative palliative radiotherapy programs.
Palliative radiotherapy: indications, benefits, side effects
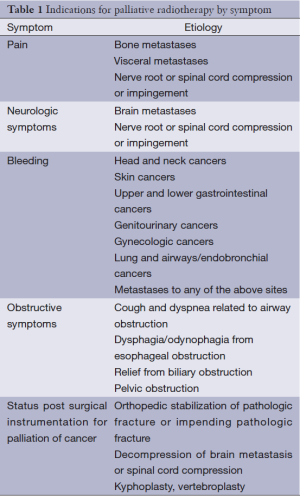
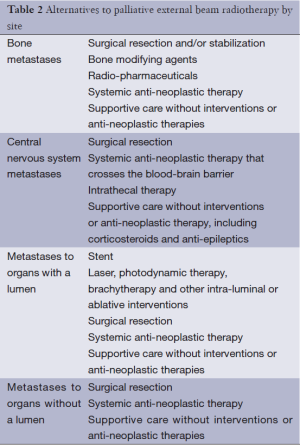
Palliative radiotherapy can be an effective treatment in any location in which a local tumor is causing symptoms for a patient. Table 1 outlines indications for palliative radiotherapy by symptom. Each of these symptoms is potentially amenable to treatment with a course of palliative radiotherapy. As with any treatment for advanced cancer, the treatment modality selected must balance the potential treatment efficacy with burdens to patient and family. A discussion of palliative radiotherapy with patients and families must, of necessity, include a discussion of alternative palliative approaches, many of which are outlined in great detail in this issue of Annals of Palliative Medicine (APM) (8). A list of common alternatives to palliative radiotherapy for specific circumstances is included in Table 2. In addition to considering alternatives to palliative radiotherapy, radiation oncologists also must decide upon the most appropriate dose-fractionation scheme for each patient and each clinical scenario. Many studies have demonstrated that short courses of radiotherapy are equivalent to longer courses of radiotherapy in terms of immediate symptom relief. However, there is a potential trade-off between these hypofractionated courses that deliver high doses per fraction with short overall treatment times when compared with more prolonged courses that deliver lower doses per fraction with more prolonged treatment times. Basic radiobiology demonstrates that a higher total dose of radiation therapy is more likely to eradicate a tumor; in addition, higher doses per fraction (hypofractionation) is more likely to cause long-term radiation side effects (9). Thus, a basic conflict within palliative radiotherapy emerges: higher radiotherapy doses may lead to more durable control of tumor, but what is the cost to time required for patient treatment? What is the cost in long-term side effects? What is the cost in durability of treatment? With ongoing advances in systemic therapies and subsequent improvements in patient survival, consideration of these short- and long-term side effects of treatment becomes more important. Prediction of survival is critical to determining the optimal radiation dose-fractionation scheme, but this remains highly challenging in spite of multiple prognostic models for various scenarios encountered in palliative radiotherapy (10-13). The remainder of this section will explore the issues of dose-fractionation and side effects for palliative radiotherapy for bone metastases, brain metastases, visceral metastases and other indications for palliative radiotherapy.
Full table
Full table
Bone metastases
Over the past 50 years, dozens of randomized controlled trials have evaluated differences in efficacy between various dose-fractionation schemes with regard to palliative radiation therapy. Multiple systematic reviews and meta-analyses have led to the same conclusions: for uncomplicated bone metastases, a single fraction of radiotherapy (8 Gy) is equivalent in pain relief to a longer course of radiotherapy, including 20 Gy in 5 fractions, 24 Gy in 6 fractions, 30 Gy in 10 fractions and longer dose-fractionation schemes (14,15). While “uncomplicated” bone metastases are defined differently in the various clinical trials, the term “uncomplicated” bone metastasis is generally taken to mean without pathologic fracture, without spinal cord compression and potentially without neuropathic pain or soft tissue component. The analyses of uncomplicated bone metastases trials demonstrate that approximately 25-40% of patients will have complete pain relief and another 50-60% of patients will have at least partial pain relief, with overall response rates to radiotherapy in the range of 75-90%. A subset analysis of the Dutch Bone Metastasis Trial (comparing 24 Gy in 6 fractions to 8 Gy in a single fraction) found there was no difference in pain control among single fraction radiotherapy and multi-fraction radiotherapy for patients who live >1 year (16). Rates of side effects were not significantly different, rates of pathologic fracture were not significantly different, and rates of subsequent spinal cord compression were also not significantly different between single and multi-fraction radiation arms (14). In the systematic review, the only statistically significant difference between single and multi-fraction radiation for uncomplicated bone metastases was the rate of retreatment (20% in single fraction regimens versus 8% in multi-fraction regimens), although this difference may be explained, in part, by radiation oncologists’ increased willingness to retreat after a single fraction of radiation. In addition to equivalent pain response rates and higher rates of retreatment with a single fraction of palliative radiotherapy, some meta-analyses have also demonstrated the pathological fracture rate was also higher in single fraction radiotherapy arm patients (17), whereas other analyses showed only trends towards an increased risk for single fraction arm patients to develop pathological fractures (15).
Even accounting for differences in retreatment rates, single fraction radiotherapy remains more cost-effective than multi-fraction radiotherapy for uncomplicated bone metastases (18). The American Society of Radiation Oncology (ASTRO) evidence based guideline suggests no more than 10 fractions for treatment of uncomplicated bone metastases based on this data (19). A more recent randomized study demonstrated the non-inferiority of single fraction (8 Gy) versus multi-fraction (20 Gy in 5 or 8 fractions) regimens in the setting of palliative re-irradiation of uncomplicated bone metastases, though there was a trend to improved pain response in the multi-fraction arm (20).
Despite the equivalence of single versus multi-fraction radiotherapy across numerous studies and patient populations, adoption of single fraction radiotherapy has been slow in many geographic areas around the world. Multiple patterns of practice studies, including international studies and recent studies from Japan and Korea, have demonstrated ongoing reluctance to utilize single fraction radiotherapy for uncomplicated bone metastases (21-23). In the United States, a recent insurance claims-based analysis demonstrated that, among more than 3,000 men with prostate cancer treated with palliative radiation for bone metastases, only 3.3% received a single fraction of radiotherapy while more than 50% received more than 10 fractions of radiotherapy (24). Among a cohort of 1,574 patients with metastatic non-small cell lung cancer who were followed prospectively, 194 patients received palliative radiotherapy to bone, but only 6% of that group received single fraction radiotherapy and only 20% received five or fewer fractions (25). Thus, one significant opportunity for collaboration among medical oncologists, radiation oncologists and palliative care clinicians is in referring patients with painful, uncomplicated bone metastases for radiotherapy earlier with the goal of increasing utilization of single fraction radiotherapy. Such change in practice is possible, as evidenced by the rapid access palliative radiotherapy clinics that have been utilized in Canada (26).
The optimal dose-fractionation scheme for complicated bone metastases (pathologic fracture, cord compression, etc.) has not been studied in a randomized controlled trial. Data from the uncomplicated bone metastases studies are not generally extrapolated to patients who have had fixation of pathologic fracture given the risk that progression of disease in the area of fixation could disrupt hardware and necessitate more surgery. As such, radiotherapy dose-fractionation schemes after surgery remain an area open to investigation, and many providers currently employ 10-fraction regimens in these patients.
The optimal treatment regimen for patients with epidural spinal cord compression requires individualization of treatment. While a randomized trial has analyzed the role of surgical decompression followed by post-operative radiotherapy and demonstrated the benefit of surgery in terms of short- and long-term ambulatory status and functional outcome, this trial was limited to patients with a single area of spinal cord compression and less than 48 hours of paraplegia (27). For patients unable to undergo surgery or for patients with longer duration of symptoms or multiple areas of spinal cord compression, other randomized controlled trials and prospective studies demonstrate equivalent short-term palliation of symptoms, but suggest a potential benefit for dose escalation in patients with longer likely survival (28-30). Rades and colleagues have developed a prognostic score for patients with spinal cord compression that incorporates six prognostic factors including tumor type, interval between diagnosis and spinal cord compression, other bone or visceral metastases, ambulatory status and duration of motor deficits (31,32). The sum of the scores ranges from 20 to 45 [see Ref (31) for details] and can be utilized to predict six month survival and to help with decision-making about which patients would benefit from short courses of radiotherapy or supportive care without radiotherapy and which patients might benefit from longer courses of radiotherapy (or surgery).
The role of advanced techniques in radiotherapy [intensity modulated radiation therapy (IMRT), stereotactic radiotherapy (SRT)] for bone metastases, including spine metastases, will be discussed separately below.
Brain metastases
The management of brain metastases has undergone a revolution since whole brain radiotherapy was first introduced in the 1950s. Early clinical trials explored various dose-fractionation schemes for whole brain radiotherapy, similar to those described for bone metastases above. In 1981, Borgelt and colleagues published a study that compared outcomes of 1 or 2 fractions of whole brain radiation (10 Gy in 1 fraction or 12 Gy in 2 fractions) with longer dose-fractionation schemes (20 Gy in 5 fractions or more) and found that, while short-course radiotherapy has equivalent short-term benefit, the durability and side effects of therapy were worse with short course radiotherapy (33). As such, whole brain radiotherapy delivered in fewer than 5 fractions is rarely indicated.
Changes in imaging (MRI), surgical techniques and radiotherapy techniques have dramatically changed the treatment options available for patients with brain metastases. The most recent ASTRO guidelines for management of brain metastases recommend utilizing a prognostic score and suggested the diagnosis-specific graded prognostic index (DS-GPA) to predict life expectancy and tailor management of brain metastases appropriately to anticipated survival (34). The DS-GPA was generated from data from nearly 4,000 patients treated on various Radiation Therapy Oncology Group (RTOG) clinical trials between 1985 and 2007 to update the recursive partitioning analysis (RPA) that had been developed in an earlier era (35). The DS-GPA utilizes factors significant for prediction of survival for specific cancer histologies, with scores ranging from 0 to 4.0 and median survival estimates ranging from 2.8 to 25.3 months (36). The factors that contribute to the DS-GPA scores include performance status, age, number of brain metastases, extra-cranial metastases and sub-type for breast cancer patients. Based on anticipated survival, treatment options can include corticosteroids alone, surgical resection, whole brain radiotherapy, radiosurgery/SRT (pinpoint high-dose radiotherapy that utilizes a coordinate system to target radiation) or supportive care without anti-neoplastic intervention.
Multiple randomized phase III trials have compared different combinations of surgery, radiosurgery and whole brain radiotherapy with or without radiation sensitizers and chemotherapy (34). For specific populations with good prognosis, surgery or radiosurgery may improve survival as compared to whole brain radiotherapy alone, particularly in patients with a single brain metastasis and with well controlled extracranial disease (37). The addition of whole brain radiotherapy to surgery or radiosurgery may improve control of tumors distant to the site of surgery or radiosurgery and may improve neurologic function, but it does not improve overall survival. Radiosensitizers and cytotoxic chemotherapies have not been shown to improve clinical outcomes or survival and may worsen symptoms, so combinations of radiosensitizers and chemotherapy with whole brain radiotherapy are not routinely recommended. Current areas of exploration in palliative radiotherapy for brain metastases include using advanced radiotherapy techniques [radiosurgery, hippocampal-sparing IMRT (38)] as a way to avoid up front whole brain radiotherapy and potentially spare patients neuro-cognitive side effects of whole brain radiation. Other groups are currently assessing dose painting strategies for whole brain radiotherapy, in which the whole brain is treated to a lower dose per day while the sites of intracranial metastases are treated to a higher dose each day (39). Questions also still remain about the role of whole brain radiotherapy in patients with poor prognoses and brain metastases. The QUARTZ trial is ongoing and compares whole brain radiotherapy (2,000 cGy in 5 fractions) versus supportive care without radiotherapy. An interim analysis has not shown a significant benefit to whole brain radiotherapy, though the study continues accrual (40).
Primary lung tumors, lung metastases, visceral metastases
Palliative radiotherapy for visceral tumors, including primary and metastatic lung cancers, primary and metastatic liver cancers and other primary and metastatic tumors is not as well studied as palliative radiotherapy is for bone and brain metastases. While randomized controlled trials have been conducted, the majority of the studies evaluating palliative radiotherapy for visceral metastases are based on small prospective and retrospective studies examining different dose-fractionation schemes for symptom palliation. A systematic review of palliation of dyspnea, cough and other symptoms related to airway compression for lung tumors demonstrated equal symptom palliation with a single fraction (10 Gy), two fractions (17 Gy in 2 fractions) and longer courses, but duration of symptom palliation and overall survival were longer in patients treated with longer dose fractionation schemes with an equivalent of 30 Gy in 10 fractions or higher doses (41). Similarly, palliation of patients with advanced hepatocellular carcinoma or metastatic disease in the liver may benefit from palliative radiation to the liver. Effective doses range from 8 Gy in a single fraction to the entire liver for patients with short life expectancy (based on a phase II study) (42) to the use of stereotactic body radiotherapy to one or multiple liver metastases either for symptomatic palliation or to provide local control to limited sites of metastases (43). Palliative radiotherapy may also provide relief from obstructive symptoms including obstruction from advanced head and neck cancer, esophageal obstruction, rectal or bladder obstruction and even biliary obstruction (44).
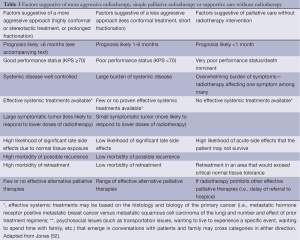
There are few randomized trials to guide selection of dose-fractionation schemes for patients with visceral metastases, but higher doses using more prolonged fractionation regimens of 10 fractions or more are more likely to provide more durable control for patients with longer life expectancy. One dose fractionation scheme that can provide effective palliation while minimizing side effects and patient/family burden has been tested with both palliative radiation for head and neck cancers and for symptomatic advanced pelvic malignancies has been described as “quad shot” radiotherapy, consisting of four fractions of hypofractionated radiation (350 to 400 cGy per fraction) delivered twice daily for 2 days (45-49). Such a hypofractionated regimen is particularly ideal for providing rapid relief to a large and symptomatic neck node or to controlling vaginal bleeding faster than more mild hypofractionation regimens would allow. Alternative split course regimens include 400 cGy daily for 5 days repeated (50). Depending on response and clinical status, these regimens may be repeated monthly to improve durability of symptom relief. Lutz and colleagues further explored the topic of when palliative radiotherapy may be worthwhile and when supportive care without radiotherapy may be optimal (51). Table 3 provides a broad overview of the factors that can be helpful in selecting an appropriate dose-fractionation scheme for patients with metastatic disease to liver, lung or other sites of visceral tumor causing symptoms.
Full table
Advanced technology in palliative radiotherapy
An understanding of when advanced radiotherapy techniques may be both efficacious and cost-effective in palliative radiotherapy requires a basic background in the various techniques involved in radiotherapy. See Table 4 for common questions regarding terminology in radiotherapy. Conventional radiotherapy with two dimensional (2D) planning utilizes simple beam arrangements, often with opposed anterior and posterior fields, to treat tumors. This treatment technique is quick to set up and has low rates of side effects for areas treated with short course schemes such as 8 Gy in a single fraction. However, treating with open fields exposes larger amounts of tissue, including normal tissues, to high doses of radiotherapy. Three dimensional (3D) planning with CT simulation allows more complex beam arrangements, increasing the conformality of the high dose radiation region while potentially spreading out the areas of low dose radiation in an attempt to reduce treatment toxicity. A further step in radiotherapy complexity utilizes modulation of radiotherapy beams (intensity-modulated radiation therapy, termed IMRT) or arc radiotherapy, allowing even higher conformality of high dose regions, including irregular shapes to avoid normal structures. SRT can be delivered to the body (termed stereotactic body radiotherapy or stereotactic ablative radiotherapy) or the brain (termed stereotactic radiosurgery) with more intensive immobilization of the patient (using a frame or full body mold). Stereotactic treatments use fiducials or other markers to allow the treated area to be visualized before and after treatment. This pinpoint radiotherapy may be utilized to treat areas with high dose radiation with rapid dose fall-off immediately adjacent, potentially allowing high doses of radiotherapy to be delivered with minimal radiation doses to surrounding normal structures. This can lead to higher biological effective doses of irradiation, and thus improvements in tumor control, as well as fewer side effects to structures outside of the high dose region. Proton therapy is an alternative form of radiotherapy that utilizes charged particles (protons) to allow the majority of the dose to be delivered at the Bragg peak, without exit dose beyond this point. While the technical details of each type of therapy is beyond the scope of this manuscript, the potential for increased dose conformality of each of these advanced techniques or therapy types offers potential dosimetric advantages over conventional 2D or 3D radiotherapy for patients referred for palliative radiotherapy: namely, with advanced technologies, high doses of irradiation may be delivered to the target volume with more limited doses to surrounding normal structures, allowing local tumor control and symptomatic relief with minimal side effects.

Full table
If the goal of palliative radiotherapy is to provide durable symptomatic relief or prevent development of symptoms, when are advanced technologies in palliative radiotherapy warranted? As described previously, the majority of patients will get clinical benefit from 2D or 3D conformal radiotherapy with moderate doses of radiation (8 Gy in a single fraction to 30 Gy in 10 fractions or 35-37.5 Gy in 14-15 fractions), and these treatments are often associated with minimal side effects. Thus, for the majority of patients, IMRT, SRT, and other advanced techniques are not necessary to achieve palliation. However, there are a number of ongoing studies that seek to evaluate questions about improving efficacy and durability of radiation therapy or minimizing side effects of treatment. RTOG 0631 is a randomized phase II-III study of patients with uncomplicated spine bone metastases without cord compression who are being randomized to single fraction stereotactic body radiotherapy to 16 or 18 Gy versus conventional radiotherapy in a single fraction of 8 Gy with a primary endpoint of complete pain relief and secondary endpoints including rapidity and durability of pain control, as well as short- and long-term side effects (53). This study could potentially change the treatment paradigm for patients with uncomplicated bone metastases with algorithms akin to those utilized in patients with brain metastases. Another area in which advanced techniques are being utilized more frequently is the re-irradiation setting, particularly when there are concerns about normal tissue tolerance of radiotherapy dose (54). In these circumstances, SRT or IMRT are able to avoid critical structures to limit side effects of radiation while still providing palliative benefit. Similarly, early results of the phase II RTOG 0933 study suggest that advanced techniques using hippocampal-sparing IMRT can preserve memory function in patients treated with whole brain radiotherapy without increasing risk of recurrence (55). Other questions remain regarding the use of post-operative radiosurgery rather than whole brain radiotherapy to decrease neurocognitive side effects of radiotherapy, again exploring whether conformal treatment may be utilized to allow patients to get the benefits of local tumor control from radiation therapy while minimizing side effects. Another area of ongoing exploration involves the treatment of oligometastatic disease (generally less than five sites of metastatic disease) with SRT, often to metastases in liver, lung, bone and brain (56). Such treatment of oligometastatic disease may or may not include patients who have symptomatic sites of metastases, although the treatment of oligometastatic disease to improve progression free survival and potentially overall survival is beyond the scope of this review.
Implementing a palliative radiotherapy program
Palliative radiotherapy can often play a role in alleviating symptoms for patients with advanced cancer. Questions remain of how best to integrate palliative radiotherapy into standard oncology care and palliative oncologic care and medical management for patients with advanced disease. While all radiation oncologists are able to deliver radiotherapy with palliative intent, there may be benefit to developing clinics and programs that specifically address the needs of patients referred for palliative radiotherapy. Multiple models have been described that allow integration of palliative radiotherapy into broader palliative oncologic care. In 1996, Chow and colleagues developed a rapid access palliative radiotherapy clinic that has decreased wait time for radiotherapy and allowed systematic focus on management of patients referred for palliative radiation. The palliative radiotherapy program has been modeled at multiple other institutions around Canada and continues to be clinically productive while furthering the understanding of palliative radiation therapy (57). The rapid access palliative radiotherapy model has been built upon by other groups, with more support for patients and families from palliative care team members at various institutions (58-60). Integration of palliative care into radiation oncology allows for more in-depth multi-disciplinary assessment and management of patients. Other novel programs are exploring more rapid treatment planning and access to allow patients to be seen and begin treatment more quickly and with less discomfort (61,62). Some programs are taking this rapid planning approach to allow integration of high dose and highly conformal radiotherapy into a single planning and treatment session to combine advanced radiotherapy techniques with palliative care clinics while studying outcomes to determine if such highly conformal radiotherapy might improve upon conventional palliative radiotherapy treatments (63). Other programs are looking to integrate palliative radiotherapy into hospice programs to allow patients on hospice to receive radiotherapy when appropriate (64). With the American Board of Radiology accepting members participation in hospice and palliative medicine certification, there is increased potential for collaboration among fields with integration of palliative care and radiation oncology (4). While there is certainly no single model for integration of palliative care and radiation oncology, local collaboration and cooperation in patient care has the potential to improve patient referrals for palliative radiation therapy and enhance overall symptom control for patients with advanced and metastatic cancer.
Conclusions
Palliative radiotherapy is a safe, effective, time-efficient method of palliating symptoms of advanced cancer. Patients who develop symptoms, including pain, neurologic deficits, bleeding or obstruction, related to local progression of cancer could benefit from early referral for palliative radiotherapy within the context of a palliative oncology model. Advanced technologies, including SRT and intensity-modulated radiotherapy, hold promise for enhancing safe and effective palliation of symptoms in select patients with advanced malignancies. Innovative models for the provision of palliative radiotherapy, including programs that integrate palliative care with radiation oncology, hold promise for meeting patient and family needs beyond the physical symptoms palliated by radiotherapy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jones J. A Brief History of Palliative Radiation Oncology. In: Lutz S, Chow E, Hoskin P, eds. Radiation Oncology in Palliative Cancer Care. 1st ed. Texas: Wiley-Blackwell, 2013.
- Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer 1954;7:682-9. [PubMed]
- Parker RG. Palliative radiation therapy. JAMA 1964;190:1000-2. [PubMed]
- Lutz S, Lupu D, Johnstone P, et al. The influence of the newly formed hospice and palliative medicine subspecialty on radiation oncology and end-of-life care. J Am Coll Radiol 2008;5:1102-5. [PubMed]
- Lutz S, Spence C, Chow E, et al. Survey on use of palliative radiotherapy in hospice care. J Clin Oncol 2004;22:3581-6. [PubMed]
- Murphy JD, Nelson LM, Chang DT, et al. Patterns of care in palliative radiotherapy: a population-based study. J Oncol Pract 2013;9:e220-7. [PubMed]
- Simone C II, Jones J. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med 2013;2:178-88.
- Simone CB II, Jones JA. Multidisciplinary approaches to palliative oncology care. Ann Palliat Med 2014;3:126-8.
- Hall E, Giaccia A. eds. Radiobiology for the Radiologist. 7th ed. Lippincott: Williams, Wilkins, 2011:1-576.
- Krishnan M, Temel JS, Wright AA, et al. Predicting life expectancy in patients with advanced incurable cancer: a review. J Support Oncol 2013;11:68-74. [PubMed]
- Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: the TEACHH model. Cancer 2014;120:134-41. [PubMed]
- Chow E, Abdolell M, Panzarella T, et al. Predictive model for survival in patients with advanced cancer. J Clin Oncol 2008;26:5863-9. [PubMed]
- Chow E, Davis L, Panzarella T, et al. Accuracy of survival prediction by palliative radiation oncologists. Int J Radiat Oncol Biol Phys 2005;61:870-3. [PubMed]
- Chow E, Zeng L, Salvo N, et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112-24. [PubMed]
- Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007;25:1423-36. [PubMed]
- van der Linden YM, Steenland E, van Houwelingen HC, et al. Patients with a favourable prognosis are equally palliated with single and multiple fraction radiotherapy: results on survival in the Dutch Bone Metastasis Study. Radiother Oncol 2006;78:245-53. [PubMed]
- Sze WM, Shelley M, Held I, et al. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Cochrane Database Syst Rev 2004;CD004721. [PubMed]
- Konski A, James J, Hartsell W, et al. Economic analysis of radiation therapy oncology group 97-14: multiple versus single fraction radiation treatment of patients with bone metastases. Am J Clin Oncol 2009;32:423-8. [PubMed]
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [PubMed]
- Chow E, van der Linden YM, Roos D, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol 2014;15:164-71. [PubMed]
- Nakamura N, Shikama N, Wada H, et al. Patterns of practice in palliative radiotherapy for painful bone metastases: a survey in Japan. Int J Radiat Oncol Biol Phys 2012;83:e117-20. [PubMed]
- Chung Y, Koom WS, Ahn YC, et al. A survey of patterns of practice on palliative radiation therapy for bone metastasis in Korea. J Cancer Res Clin Oncol 2013;139:2089-96. [PubMed]
- Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys 2009;75:1501-10. [PubMed]
- Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA 2013;310:1501-2. [PubMed]
- Chen AB, Cronin A, Weeks JC, et al. Palliative radiation therapy practice in patients with metastatic non-small-cell lung cancer: a Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) Study. J Clin Oncol 2013;31:558-64. [PubMed]
- Wu JS, Kerba M, Wong RK, et al. Patterns of practice in palliative radiotherapy for painful bone metastases: impact of a regional rapid access clinic on access to care. Int J Radiat Oncol Biol Phys 2010;78:533-8. [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [PubMed]
- Maranzano E, Trippa F, Casale M, et al. 8Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol 2009;93:174-9. [PubMed]
- Maranzano E, Bellavita R, Rossi R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol 2005;23:3358-65. [PubMed]
- Rades D, Douglas S, Veninga T, et al. A survival score for patients with metastatic spinal cord compression from prostate cancer. Strahlenther Onkol 2012;188:802-6. [PubMed]
- Rades D, Douglas S, Veninga T, et al. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer 2010;116:3670-3. [PubMed]
- Rades D, Dunst J, Schild SE. The first score predicting overall survival in patients with metastatic spinal cord compression. Cancer 2008;112:157-61. [PubMed]
- Borgelt B, Gelber R, Larson M, et al. Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1981;7:1633-8. [PubMed]
- Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012;2:210-25. [PubMed]
- Gaspar LE, Scott C, Murray K, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys 2000;47:1001-6. [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-9. [PubMed]
- Oskan F, Ganswindt U, Schwarz SB, et al. Hippocampus sparing in whole-brain radiotherapy. A review. Strahlenther Onkol 2014;190:337-41. [PubMed]
- ClinicalTrials.gov. 2014. Available online: http://clinicaltrials.gov/ct2/show/NCT01414738?term=brain+metastasis+radiotherapy&rank=12
- Langley RE, Stephens RJ, Nankivell M, et al. Interim data from the Medical Research Council QUARTZ Trial: does whole brain radiotherapy affect the survival and quality of life of patients with brain metastases from non-small cell lung cancer? Clin Oncol (R Coll Radiol) 2013;25:e23-30. [PubMed]
- Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol 2008;26:4001-11. [PubMed]
- Soliman H, Ringash J, Jiang H, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 2013;31:3980-6. [PubMed]
- Høyer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys 2012;82:1047-57. [PubMed]
- Konski A, Feigenberg S, Chow E. Palliative radiation therapy. Semin Oncol 2005;32:156-64. [PubMed]
- Ghoshal S, Chakraborty S, Moudgil N, et al. Quad shot: a short but effective schedule for palliative radiation for head and neck carcinoma. Indian J Palliat Care 2009;15:137-40. [PubMed]
- Spanos WJ Jr, Clery M, Perez CA, et al. Late effect of multiple daily fraction palliation schedule for advanced pelvic malignancies (RTOG 8502). Int J Radiat Oncol Biol Phys 1994;29:961-7. [PubMed]
- Caravatta L, Padula GD, Macchia G, et al. Short-course accelerated radiotherapy in palliative treatment of advanced pelvic malignancies: a phase I study. Int J Radiat Oncol Biol Phys 2012;83:e627-31. [PubMed]
- Cameron MG, Kersten C, Guren MG, et al. Palliative pelvic radiotherapy of symptomatic incurable prostate cancer - a systematic review. Radiother Oncol 2014;110:55-60. [PubMed]
- van Lonkhuijzen L, Thomas G. Palliative radiotherapy for cervical carcinoma, a systematic review. Radiother Oncol 2011;98:287-91. [PubMed]
- Kancherla KN, Oksuz DC, Prestwich RJ, et al. The role of split-course hypofractionated palliative radiotherapy in head and neck cancer. Clin Oncol (R Coll Radiol) 2011;23:141-8. [PubMed]
- Lutz S, Korytko T, Nguyen J, et al. Palliative radiotherapy: when is it worth it and when is it not? Cancer J 2010;16:473-82. [PubMed]
- Jones J. Too much, too little, or just the right amount: finding the balance in palliative radiotherapy. Curr Probl Cancer 2011;35:325-36. [PubMed]
- RTOG website. 2014. Available online: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0631
- Mantel F, Flentje M, Guckenberger M. Stereotactic body radiation therapy in the re-irradiation situation--a review. Radiat Oncol 2013;8:7. [PubMed]
- Gondi V, Mehta M, Pugh S, et al. Memory Preservation with Conformal Avoidance of the Hippocampus During Whole-Brain Radiation Therapy for Patients with Brain Metastases: Primary Endpoint Results of RTOG 0933. Int J Radiat Oncol 2013;87:1186.
- Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14:e28-37. [PubMed]
- Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer 2006;14:38-43. [PubMed]
- Bansal M, Patel FD, Mohanti BK, et al. Setting up a palliative care clinic within a radiotherapy department: a model for developing countries. Support Care Cancer 2003;11:343-7. [PubMed]
- Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer 2009;17:163-70. [PubMed]
- Pituskin E, Fairchild A, Dutka J, et al. Multidisciplinary team contributions within a dedicated outpatient palliative radiotherapy clinic: a prospective descriptive study. Int J Radiat Oncol Biol Phys 2010;78:527-32. [PubMed]
- Wong RK, Letourneau D, Varma A, et al. A one-step cone-beam CT-enabled planning-to-treatment model for palliative radiotherapy-from development to implementation. Int J Radiat Oncol Biol Phys 2012;84:834-40. [PubMed]
- Samant R, Scopazzi M, Carty K. Assessing patient outcomes after palliative radiotherapy using image-guided intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:e125. [PubMed]
- David Wilson, Ke Sheng, Wensha Yang, et al. STAT-RAD: A Potential Real-Time Radiation Therapy Workflow. In: Natanasabapathi G. eds. Modern Practices in Radiation Therapy. Rijeka: InTech, 2012:1-19.
- Schuster J, Coyne P, Lutz S, et al. Consultation and Delivery of Palliative Radiotherapy in a Single Day for Cancer Patients Enrolled in Hospice (S755). J Pain Symptom Manage 2013;45:451-2.


