Targeted therapy for lung cancer: present and future
Introduction
Over the last decade, the oncology field has witnessed significant advances in our understanding of biology of oncologic diseases. Instead of treating a particular histology, or organ system, it is now clear that a cancer with a specific histology represents a constellation of diseases with distinct molecular profiles and sensitivities to treatment. This has largely been boosted by the availability of genomic and transcriptomic profiling, and accessibility of high-throughput and cost-effective readouts of hundreds of individual mutations affecting dozens of cancer genes. Personalized therapy based on patient’s individual biologic and molecular profile is a promising approach to optimize efficacy with the available agents. This understanding has allowed us to deliver targeted therapy, which utilizes agents that affect a known aberrant pathway or molecular target in the cancer cell or tumor microenvironment. Well-documented examples of targeted therapy include the use of trastuzumab to treat HER2-amplified breast cancer (1) and imatinib for BCR-ABL translocated chronic myelogenous leukemia (2). Over the last decade, we have seen an increase in clinical trials of drugs targeting oncoproteins and cancer pathways in various solid tumors. Prominent examples include agents targeting receptor tyrosine kinases (TK), mitogen-activated protein kinase pathway proteins, phosphoinositol-3 kinase (PI3K) pathway components, and the Janus kinase (JAK)—signal transducer and activator of transcription (STAT) pathway. Based on promising activity and improved outcomes, numerous targeted agents have been approved across several disease types. Well-known examples include erlotinib/gefitinib (3) and crizotinib (4) for patients with activating mutations in the epidermal growth factor receptor (EGFR) domain and echinoderm microtubule associated protein-like 4- anaplastic lymphoma kinase (EML4-ALK) translocated non-small cell lung cancer (NSCLC) respectively, vemurafenib for melanoma with BRAF V600E mutation (5), lapatinib for HER2 amplified breast cancer (6) and ruxolitinib for patients with JAK-2 mutation (7).
Lung cancer is the most common cause of cancer-related death worldwide (8). NSCLC constitutes the majority of patients diagnosed with lung cancer. Approximately 40% of patients with newly diagnosed NSCLC present with advanced disease, where chemotherapy is the mainstay of treatment. Recently, molecular subtyping of NSCLC has led to approval of and use of targeted therapies in the front-line setting (Table 1). Patients with activating mutations in the EGFR domain and EML4-ALK translocation benefit from first-line treatment with erlotinib or crizotinib, respectively (3,9,10,17). These mutations are seen in a relatively small subset of NSCLC patients. They are common in patients with adenocarcinoma, never smokers and patients of East Asian origin. KRAS is the most common mutation found in NSCLC; however, we currently do not have an effective targeted therapy for this subset of NSCLC. Despite the addition of new therapies, the median 5-year overall survival of patients in advanced staged disease remains a dismal 1-2%.
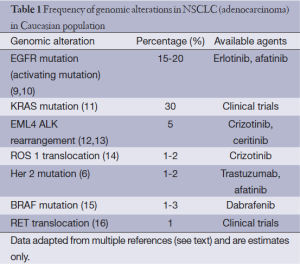
Full table
At the same time, multiple studies have shown that palliative care services improve symptoms, and when incorporated into the treatment management paradigm early, can allow patients achieve a better quality of life, avoid hospitalization. Targeted therapies allow integration of early palliative care into the management of advanced stage diseases, as these therapies are associated with better quality of life, improved symptoms and health status.
This review will focus on current state of targeted treatment approaches for advanced NSCLC, with a special emphasis on novel molecularly targeted agents, and discuss promising agents in development.
EGFR mutation
EGFR is one of a family of receptors that is over expressed in about 40-80% of NSCLC (18). Mutations in the TK domain of the EGFR receptor were first discovered and reported in 2004 (9,10,19). They are more prevalent in never-smoking patients with adenocarcinoma. In frame deletions in exon 19 and the L858R point mutation in exon 21 are the two most commonly seen activating mutations in the ATP binding pocket of the EGFR TK domain. EGFR mutations significantly predict for both an increased response to TK therapy and a favorable prognosis in patients with advanced lung adenocarcinoma, thereby making the presence of EGFR mutations a powerful prognostic and predictive marker in NSCLC (3,20-24). Erlotinib, which was originally approved in unselected patients in the second and third line setting after progression on platinum-based therapy, is now considered the standard of care for front line treatment of patients whose tumors harbor an activating mutation in the EGFR domain. Beginning with the trial conducted by Mok et al., that randomized previously untreated patients with advanced stage NSCLC with high likelihood of carrying EGFR mutations (adenocarcinoma, never or light smokers) to receive gefitinib or cytotoxic chemotherapy (3), there have been several large, phase III randomized trials that established the place of the EGFR TKIs in the treatment algorithm (3,25).
Across trials, response rates to EGFR TKI vary from 55% to 70%, however, despite this advance, unfortunately median PFS is approximately 9-10 months in most studies with the majority of patients with EGFR mutant tumors sustaining disease progression by 1 year, mostly due to development of resistance by multiple disparate pathways. Rebiopsy at the time of progression has become standard practice, to elucidate the mechanisms of resistance. Emergence of secondary EGFR mutation, T790M, which is resistant to current TKIs is the most common mechanism of resistance (26). This is presumed to develop from a resistant population of cells already present in low numbers before treatment with EGFR TKIs. Other pathways, including MET amplification, mutations in PIK3CA, mutations in BRAF, amplification of HER2, and activation of the AXL kinase, have also been implicated to confer resistance. An understanding of the biology of acquired resistance opens up prospective to develop new therapeutic paradigms.
Afatinib is an oral, irreversible ErbB family inhibitor targeting EGFR and HER2 that has shown activity in erlotinib- and gefitinib-resistant lung cancer models (27). Afatinib has been evaluated in the first line setting against standard chemotherapy (LUX-Lung 3), as well as in patients who had previously been treated with EGFR TKIs (LUX-Lung 1 and 2). LUX-Lung 1 was a randomized, double-blind, phase IIb/III study that evaluated afatinib vs. placebo plus best supportive care (BSC) in patients who had received one or two previous chemotherapy regimens and had disease progression after at least 12 weeks of treatment with erlotinib or gefitinib (28). Disease control rate at 8 weeks was higher for afatinib at 58% compared with 19% for placebo plus BSC (P<0.0001). Median progression-free survival was longer in the afatinib group (3.3 months) than it was in the placebo group [1.1 months, hazard ratio (HR) =0.38; P<0.0001]. Median overall survival, however, was not different between arms (10.8 vs. 12 months; HR =1.08; P=0.74).
LUX-Lung 2, evaluated the efficacy of afatinib in patients with advanced NSCLC harboring an EGFR-activating mutation (29). In this multicenter, phase II, open-label single-arm study, afatinib was dosed at 40 or 50 mg once daily. For 129 patients that were enrolled, an overall response rate (ORR) of 67% (confirmed ORR of 60%), median PFS of 14 months, and median OS of 24 months was noted. In patients with an exon 19 deletion or a L858R mutation, the objective RR was 69% and 59%, respectively, DCR was 93% and 83%, respectively, and PFS 13.7 and 16.1 months, respectively. Comparable efficacy was observed in the first- and second-line settings.
The LUX-Lung 3 trial compared 40 mg afatinib to intravenous pemetrexed and cisplatin (500+75 mg/m2 q21 days up to 6 cycles) as first-line therapy in 345 NSCLC patients harboring an EGFR-activating mutation. ORR was significantly higher with afatinib (56% vs. 23%; P<0.0001). Median PFS was significantly better for afatinib compared with cytotoxic chemotherapy (11.1 vs. 6.9 months; HR =0.58; P=0.0004). In 308 patients with common mutations (Del19/L858R), median PFS was 13.6 months (30). Based on these results, afatinib was approved by the FDA in August, 2013.
Several new treatments that irreversibly target EGFR family members are under development for patients with NSCLC, most in early clinical phases.
Irreversible inhibitors like neratinib (HKI-272) and dacomitinib (PF00299804) have been evaluated. Despite promising preclinical data, neratinib demonstrated marginal activity in both TKI-naïve patients and patients with prior benefit from TKIs, and was therefore discontinued from further development in NSCLC (31). Dacomitinib was studied in a randomized phase II trial in patients with advanced NSCLC previously exposed to platinum-based therapy. One hundred eighty-eight patients were randomly assigned to receive dacomitinib or erlotinib. Even though median PFS was superior [2.8 months for dacomitinib and 1.9 months for erlotinib (HR =0.66; P=0.012)], there was no statistical difference in median OS [9.5 months for dacomitinib and 7.4 months for erlotinib (HR =0.80; 95% CI, 0.56 to 1.13; 2-sided P=0.205)] (32). The overall improvement in PFS seen with dacomitinib was noted across most clinical and molecular subsets assessed. A phase III study [Advanced Research for Cancer Targeted Pan-HER Therapy (ARCHER 1009)] is underway to confirm the findings of this study for second-/third-line therapy in patients with advanced NSCLC (33).
For patients with T790M mutation, combination afatinib and cetuximab has shown promising results. Of 22 patients that were treated, 36% showed partial responses (34). Third generation EGFR TKIs such as CO-1686 have been developed that specifically inhibit the T790M mutant EGFR protein. CO-1686 is currently being tested in a phase I trial in patients with advanced EGFR-mutant NSCLC that have progressed on other EGFR TKIs, where it has shown preliminary evidence of efficacy in resistant disease and a favourable toxicity profile (35). AZD9291 is another third generation EGFR TKI with promising early data in patients with T790M.
MET, another TK, plays an important role in signaling pathways, especially as a resistance mechanism after EGFR TKI blockade. Several MET inhibitors are currently in clinical development. MetMAb is the furthest along in clinical trials. MetMAb (Onartuzumab), a monovalent, single arm monoclonal antibody that binds specifically to the extracellular domain of the MET receptor, blocks ligand mediated activation and further downstream signaling. It was evaluated in combination with erlotinib and compared to erlotinib alone in 128 erlotinib-naïve NSCLC patients whose disease had progressed on one or two prior lines of treatment. In patients with MET overexpression (MET diagnostic positive), PFS (HR =0.56; P=0.05) and OS (HR =0.55; P=0.11) were increased in favor of the combined treatment with MetMAb and erlotinib. Intriguingly, MET diagnostic negative patients receiving both MetMab and erlotinib had inferior outcomes compared to erlotinib alone. Besides MET-positive patients, no other subgroup with any clinical benefit from MetMAb was identified (36). Based on these encouraging results, a global randomized phase III trial in MET-diagnostic positive patients is currently ongoing (37).
EML4-ALK translocation
ALK is a receptor TK first identified in anaplastic large cell lymphoma. EML4-ALK is a fusion protein in which the N-terminal half of EML4 is fused to the intracellular kinase domain of ALK and leads to expression of a chimeric TK with potent oncogenic activity both in vivo and in vitro (12,38,39). This translocation causes aberrant activation of downstream oncogenic signaling pathways such as MAP kinase, PI3 kinase, and STAT, leading to cell proliferation, invasion, and inhibition of apoptosis.
In a retrospective genetic screening of 141 NSCLC tumors screened, patients with EML4-ALK mutant tumors were significantly more likely to be younger, male and never/light smokers. An overwhelming majority of the EML4-ALK tumors were adenocarcinomas, and EML4-ALK positivity was associated with resistance to EGFR TKIs (4). Histologically, signet ring features are often present. Even though EML4-ALK translocation is found in a limited subset of patients, only 3-6% of all cases of NSCLC, it constitutes 35,000-40,000 cases annually worldwide.
Crizotinib is currently approved for treatment of advanced NSCLC harboring an EML4-ALK translocation. This approval was granted based on response rates of 60% or higher observed in various early clinical studies (17,40). A phase III trial (PROFILE 1007) comparing second-line crizotinib with either pemetrexed or docetaxel in NSCLC with ALK translocation was just completed. Response rate was considerably higher in the crizotinib arm with markedly improved PFS (7.7 months) vs. the control group (3 months). Crizotinib also improved baseline symptoms and delayed subsequent worsening to a greater degree than chemotherapy in quality of life analyses. There was no overall survival benefit seen, most likely because at least 64% of patients in the chemotherapy arm subsequently received crizotinib (13).
In addition, PROFILE 1014, a randomized open-label phase III study of crizotinib vs. pemetrexed/cisplatin or pemetrexed/carboplatin in previously untreated metastatic non-squamous cell carcinoma of the lung is currently enrolling patients.
Resistance to crizotinib invariably occurs. Similar to the biology of EGFR resistance, rebiopsy at the time of progression has led to insights into mechanisms of resistance to ALK inhibitors. Mutations in the ALK gene appear to mediate resistance in around one-third of patients. Activation of alternate signalling pathways involving EGFR and c-KIT (an oncogene targeted by imatinib) may also play a role in mediating resistance. In about a third, the mechanism of crizotinib resistance currently remains unknown (41).
Several new ALK inhibitors are under development. Ceritinib (LDK378) is a novel, potent and selective small molecule ALK inhibitor, which does not inhibit c-MET. A phase I study was conducted in patients with tumors with ALK rearrangement, amplification or mutation. Patients received once daily oral ceritinib on a continuous 21-day schedule. A response rate of 81% was reported in 21/26 NSCLC patients treated at ≥400 mg whose disease had progressed following crizotinib. There was also hints of anti-tumor activity against brain metastases at the 750 mg dose (42). The most common AEs included nausea, vomiting and diarrhea (43). Other agents like CH5424802 (93% ORR) and AP26113 are also being studied in clinical trials with promising early results (44).
KRAS mutation
Kirsten rat sarcoma viral oncogene is a member of the RAS family and an important downstream signaling target in the survival pathways. Mutations in the KRAS gene are the most common mutation seen in adenocarcinomas (about 15-25%). A meta-analysis showed that the mutations were more common in adenocarcinoma than in other histologic types (P value <0.01) and in current or former smokers than in never-smokers (P value <0.01) (45). NSCLC with KRAS mutation forms a distinct subset, and remains a therapeutic challenge; we currently do not have any agents approved for use in this cohort. There has been interest in the development of inhibitors of MEK, a cell signaling pathway downstream from KRAS.
Selumetinib, (an orally available BRAF and MEK inhibitor) has been evaluated in KRAS mutant NSCLC patients who had received prior chemotherapy. On this trial, patients received docetaxel, either alone or in combination with oral selumetinib. OS was numerically longer for selumetinib (9.4 vs. 5.2 months) but did not reach statistical significance (HR =0.80; 80% CI, 0.56; P=0.2069). All secondary endpoints, including RR (37% vs. 0%; P<0.0001) and PFS (Selumetinib 5.3 vs. 2.1 months; P=0.0138), were significantly improved for selumetinib in combination with docetaxel. The combination, however, was also more toxic (11). Based on these promising early data, a phase III trial is currently underway.
Other agents that target driver oncogenes beyond EGFR, ALK and KRAS have also been characterized in NSCLC, often at frequencies of less than 5%. Crizotinib and dabrafenib have shown significant efficacy in patients with ROS-1 (14) translocations and BRAF (15) mutations, respectively (Table 1).
As we identify additional mutations, clinical trial efforts have focused on identifying and matching patients with specific genetic alterations to appropriate therapies within early phase trials.
Conclusions
This review summarizes currently approved targeted therapies and strategies for individualized treatment for patients with advanced NSCLC. In solid tumor oncology, with the availability of modern techniques for whole genome sequencing, we have gained several new insights into disease biology. This knowledge has changed the therapeutic landscape. Molecular characterization is now routinely employed to guide therapeutic decisions, and oral, small molecule inhibitors have firmly secured their place in the treatment paradigm of several solid tumors. Targeted therapies are often associated with improvement in quality of life, and are well tolerated, therefore lending themselves to be perfect partners for early integration to palliative care for management of advanced stage NSCLC.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012;4:CD006243. [PubMed]
- O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003;348:994-1004. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008;112:533-43. [PubMed]
- Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014;120:513-20. [PubMed]
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013;14:38-47. [PubMed]
- Soda M, Takada S, Takeuchi K, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A 2008;105:19893-7. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Ignatius Ou SH, Bang YJ, Camidge DR, et al. Efficacy and safety of crizotinib in patients with advanced ROS1-rearranged non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8032.
- Planchard D, Mazieres J, Riely GJ, et al. Interim results of phase II study BRF113928 of dabrafenib in BRAF V600E mutation–positive non-small cell lung cancer (NSCLC) patients. J Clin Oncol 2013;31:abstr 8009.
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378-81. [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 1995;19:183-232. [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Shepherd FA, Rodrigues Pereira J, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Takano T, Fukui T, Ohe Y, et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol 2008;26:5589-95. [PubMed]
- Zhu CQ, da Cunha Santos G, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008;26:4268-75. [PubMed]
- Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 2008;26:2442-9. [PubMed]
- Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med 2005;353:133-44. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Shih JY, Gow CH, Yang PC. EGFR mutation conferring primary resistance to gefitinib in non-small-cell lung cancer. N Engl J Med 2005;353:207-8. [PubMed]
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702-11. [PubMed]
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. [PubMed]
- Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol 2012;13:539-48. [PubMed]
- Yang JC, Schuler MH, Yamamoto N, et al. LUX-Lung 3: A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. J Clin Oncol 2012;30:abstr LBA7500.
- Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol 2010;28:3076-83. [PubMed]
- Ramalingam SS, Blackhall F, Krzakowski M, et al. Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2012;30:3337-44. [PubMed]
- Spigel DR, Edelman MJ, Mok T, et al. Treatment Rationale Study Design for the MetLung Trial: A Randomized, Double-Blind Phase III Study of Onartuzumab (MetMAb) in Combination With Erlotinib Versus Erlotinib Alone in Patients Who Have Received Standard Chemotherapy for Stage IIIB or IV Met-Positive Non-Small-Cell Lung Cancer. Clin Lung Cancer 2012;13:500-4. [PubMed]
- Janjigian YY, Groen HJ, Horn L, et al. Activity and tolerability of afatinib (BIBW 2992) and cetuximab in NSCLC patients with acquired resistance to erlotinib or gefitinib. J Clin Oncol 2011;29:abstr 7525.
- Sequist LV, Soria JC, Gadgeel SM, et al. First-in-human evaluation of CO-1686, an irreversible, selective, and potent tyrosine kinase inhibitor of EGFR T790M. J Clin Oncol 2013;31:abstr 2524.
- Spigel DR, Ervin TJ, Ramlau R, et al. Final efficacy results from OAM4558g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC. J Clin Oncol 2011;29:abstr 7505.
- Spigel DR, Edelman MJ, Mok T, et al. Treatment Rationale Study Design for the MetLung Trial: A Randomized, Double-Blind Phase III Study of Onartuzumab (MetMAb) in Combination With Erlotinib Versus Erlotinib Alone in Patients Who Have Received Standard Chemotherapy for Stage IIIB or IV Met-Positive Non-Small-Cell Lung Cancer. Clin Lung Cancer 2012;13:500-4. [PubMed]
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Crinò L, Kim D, Riely GJ, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol 2011;29:abstr 7514.
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [PubMed]
- Mehra R, Camidge DR, Sharma S, et al. First-in-human phase I study of the ALK inhibitor LDK378 in advanced solid tumors. J Clin Oncol 2012;30:abstr 3007.
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [PubMed]
- Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 2013;14:590-8. [PubMed]
- Mao C, Qiu LX, Liao RY, et al. KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies. Lung Cancer 2010;69:272-8. [PubMed]


