
Elevated plasma histone H4 level predicts increased risk of mortality in patients with sepsis
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to an infection (1). A histone is an intra-nuclear structural protein that usually presents at very low levels in the peripheral circulating blood (2). A significant elevation of histones in the plasma and serum can occur when they are released into the blood in response to cell death, such as in sepsis (3,4). Both laboratory and clinical studies have suggested that histones have an essential role in the pathogenesis of sepsis (5-11). Extracellular histones, mainly H4 and H3, could accelerate septic progression by mediating direct injury on the endothelial cells (4,11,12). The infusion of histone antibodies could effectively reduce the mortality rate of sepsis mice (8,9). This study showed that histones played a vital role in the occurrence and development of sepsis. Therefore, we hypothesize that they are a potential target for the treatment of sepsis.
Ekaney et al. (11) also investigated the role of histone H4 and found that higher histone levels were significantly associated with an increased mortality rate in septic patients. However, the confounded factors were not considered and the small sample size in their study limited its clinical acceptance, and more studies are needed.
The present study investigated whether plasma histone H4 could be an effective biomarker to assess the severity of sepsis based on the binary logistic model and a receiver operating characteristic (ROC) curve. Also, we evaluated associations between histone H4 levels and other biochemical indicators of sepsis. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1011).
Methods
Study design and participants
This prospective observational study was conducted between May 2016 and January 2019 in the Department of Critical Care Medicine at Subei People’s Hospital of Jiangsu Province with 35 intensive care unit (ICU) beds. The study protocol was approved by the Hospital Ethics Committees and was performed in full compliance with the Declaration of Helsinki. All the study participants, or their health care proxies, supplied informed consent.
The patients with sepsis were enrolled at once after admission to the ICU. Sepsis was diagnosed in all the patients (n=126) based on the Sepsis-3.0 criteria of the Third International Consensus Definitions Task Force [sepsis = infection + sequential organ failure assessment (SOFA) score ≥2] (13). Patients with any of the following were excluded from this study: <18 or >80 years; abnormal segmental ventricular wall movement (i.e., possible acute or previous myocardial infarction); pregnancy; length of ICU stay <24 hours; a history of chronic heart failure, dilated or hypertrophic obstructive cardiomyopathy, or heart valve disease.
The patients with sepsis were compared to 10 healthy volunteers (control group) concerning plasma histones, cardiac troponin I (TnI), N-terminal pro-b-type natriuretic peptide (NT-proBNP), procalcitonin (PCT), and lactate. The healthy volunteers were aged between 23 and 26 years and recruited from postgraduates of the same medical university where their health status had been assessed previously.
Study protocol
The following information was documented for each patient and control individual: age; gender; weight; height; site of infection; duration of ICU stay; use of vasopressor; and plasma histone H4, cardiac TnI, NT-proBNP, PCT, and lactate. SOFA scores were calculated at 24 hours after admission. Survival or death during hospitalization was recorded.
All patients received appropriate Surviving Sepsis Campaign-guided treatments as determined by their primary ICU team (14). Treatment included antibiotics, fluid resuscitation, vasopressors, and ventilator support, if necessary.
Blood samples were collected into EDTA tubes (Sainty International Group Jiangsu Yangzhou Sumex Imp. & Exp. Co., Ltd., China) within 24 hours after patients were admitted to the ICU. Supernatants were obtained after centrifugation at 3,000× rpm for 10 minutes and were frozen at −80 °C within half an hour for later analysis. All blood tests were analyzed following the instructions of the manufacturers (Beijing Leagene Biotech, China) at the hospital laboratory. For measuring histone H4, both standards and test wells were prepared. In each standard well, 50 µL of the standard solution was added. In each test well, 10 µL of the test sample and 40 µL of sample diluent were added. A blank well was also prepared. In each standard well and test well, 100 µL of horseradish peroxidase-conjugated reagent was added. These wells were then covered with an adhesive strip and incubated for 60 minutes at 37 °C. Each well was aspirated and washed with wash solution (400 µL) four times. After the last wash, any remaining wash solution was removed. To each well, 3,3'-diaminobenzidine substrate chromogen solution (50 µL) and 3% aquae hydrogenii dioxide substrate chromogen solution B (50 µL) was added, and then gently mixed and incubated for 15 minutes at 37 °C in the dark. The optical density was measured at a wavelength of 450 nm using a microtiter plate reader.
Statistical analysis
Continuous data are presented as mean ± standard deviation and were compared by t-test or Wilcoxon rank-sum test, when proper. Categorical data are presented as frequency or percentage and compared using the chi-squared test. Spearman’s correlation analysis was applied to study the associations between plasma histone H4 level and other variables. Binary logistic analysis was used to study the value of different variables in predicting mortality from sepsis. A ROC curve was created to evaluate the prognostic value of histone for mortality due to sepsis. P<0.05 was considered statistically significant. All analyses were performed with SPSS (version 22.0, IBM, USA) software.
Results
Patient enrollment and comparisons to healthy controls
From May 2016 to January 2019, 198 patients with sepsis or septic shock were initially selected for this study. Seventy-two patients were excluded for distinct reasons. Finally, 126 patients who fulfilled the inclusion criteria were enrolled. Compared to the healthy controls, patients with sepsis had significantly higher levels of histones, cardiac TnI, and NT-proBNP (Table 1).
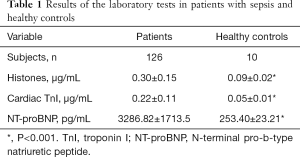
Full table
Survival and death
Among the 126 patients, 37 died during the ICU stay. Between those who survived and those who died, the following characteristics were comparable: age, gender, body mass index, and the number of infection sites (Table 2). However, compared with the patients who survived, those who died had significantly higher SOFA scores, plasma histone H4 levels, cardiac TnI, and more frequent vasopressor administrations within the first 24 hours after ICU admission (Table 3).
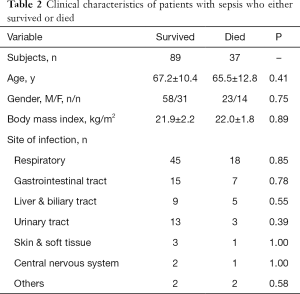
Full table
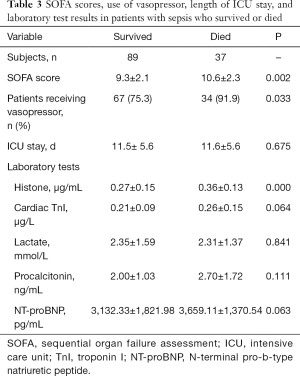
Full table
Binary logistic analysis to predict independent risk factors of death in septic patients
The multivariate logistic analysis showed that high plasma histone level was an independent risk factor for mortality in septic patients (Table 4).
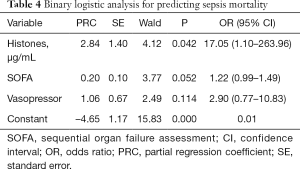
Full table
Correlation between plasma histones and other variables
Spearman’s linear correlation analysis showed that the plasma histone H4 level correlated significantly and positively with SOFA score, cardiac TnI, NT-proBNP, and lactate levels (Table 5). However, the significance of correlations with the following was only moderate: SOFA score, cardiac TnI, and blood lactate. The histone H4 was weakly associated with NT-proBNP and PCT.
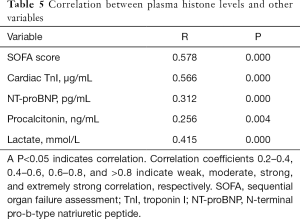
Full table
Comparisons between patients with higher and lower levels of plasma histone H4
Compared to patients with a lower plasma histone level (<0.3 µg/mL), patients with a higher plasma histone level (≥0.3 µg/mL) had significantly higher SOFA scores, mortality, frequency of vasopressor administration, and plasma levels of cardiac TnI, NT-proBNP, lactate, and PCT. For patients in the low and high plasma histone groups, the duration of ICU stay was similar (Table 6).
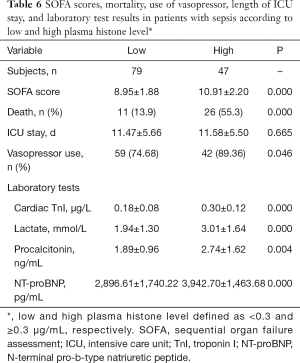
Full table
ROC curve analyses
When the cutoff point was set as 0.30 µg/mL for plasma histone H4, prediction of mortality had a sensitivity of 70.3% and a specificity of 76.4% (P<0.05), with an area under the ROC curve (AUC) of 0.731 (Figure 1). When the cutoff point was set as 9.5 for the SOFA score, the prediction of death had a sensitivity of 73% and a specificity of 58.4% (P<0.05), with an AUC of 0.67.
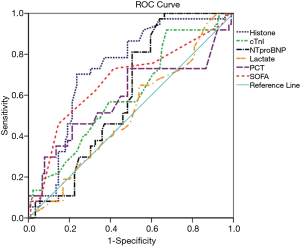
The predictive values of cardiac TnI, NT-proBNP, lactate, and PCT were low and insignificant (Figure 1). Specifically, with a cutoff point of 0.15µg/mL, the prediction of mortality of cardiac TnI had a sensitivity of 91.9%, the specificity of 32.6%, and the AUC of 0.605. With a cutoff point of 2,152.50 pg/mL, the prediction of mortality of NT-proBNP had a sensitivity of 97.3%, the specificity of 36%, and the AUC of 0.605. Lactate, at a cutoff point of 1.78 mmol/L, had a sensitivity of 64.9%, the specificity of 46.1%, and the AUC of 0.511. PCT, at a cutoff point of 1.60 ng/mL, had a sensitivity of 73%, the specificity of 51.7%, and the AUC of 0.59.
Discussion
In this study, the mean plasma histone level of patients with sepsis was significantly higher than in the control group. Furthermore, the histone levels of the patients who died during hospital admission were significantly higher than the patients who survived.
The mechanisms of histone elevation in sepsis are not well understood. Histones are proteins in eukaryotic cells that are specialized to bind to the DNA to form chromatin. Studies have shown that, under certain conditions of sepsis, neutrophils formed so-called extracellular traps consisting of a complex composed of DNA, nuclear proteins, and histones, and important innate antimicrobial components (15). Also, histones make up significant components of the nucleosomes (15). Furthermore, cell apoptosis and cell death due to sepsis have been associated with a large number of nucleosomes released into the peripheral circulation (16).
Correlation between plasma histones and cTnI in patients with sepsis
Cardiac TnI, a widely accepted marker for myocardial injury, mainly stays within myocardial cells and can increase in patients with sepsis (17). This is probably due to microdamage of myocardial cells induced by sepsis, and other factors such as cytokines released during the systemic inflammatory response. Studies have shown that histones can damage myocardial cells, with subsequent release of cardiac TnI into the circulating blood (18,19). The present study showed that levels of plasma cardiac TnI positively correlated with plasma histone H4 levels.
Correlation between plasma histone H4 and NT-proBNP in patients with sepsis
NT-proBNP is widely used to assess heart failure and evaluate patients’ prognosis. The usage is a result of it having a long half-life, in vitro stability, and is easier to detect compared with B-type natriuretic peptide (BNP). Studies have shown NT-proBNP may be used as a biomarker to evaluate prognosis in patients with sepsis (20,21).
In the present study, the plasma NT-proBNP levels of patients with sepsis were significantly higher than the healthy volunteers, and plasma histone H4 and NT-proBNP levels correlated weakly. Increased NT-proBNPs in sepsis is due to increased tensions in the ventricular wall, while elevated histone is mainly caused by myocardial damage via various factors. The low strength of the correlation between NT-proBNP and histones may be due to the differences in the underlying mechanisms responsible for their elevations.
The ROC analysis showed that the AUC value for plasma histone H4 levels was above 0.731, while that for NT-proBNP was below 0.70. These findings show that histone level was a better predictor of mortality rate than NT-proBNP in these patients with sepsis.
Correlation between plasma histones and lactate in patients with sepsis
Blood lactate is a standard indicator of organ dysfunction and sepsis severity. A high lactate level was reported in critically ill patients (22,23). However, some authors have suggested that the absolute value of blood lactate did not correctly reflect the prognosis of these patients (24), and blood lactate clearance may be a better indicator to monitor patient prognosis (25,26). In the current study, plasma histones and lactate levels only had a moderate positive correlation. However, the ROC curve analysis showed that plasma lactate was not a good predictor of death. In regard to plasma lactate and histones, further research is needed to determine whether the progression of sepsis and treatment response can be monitored via changes in the plasma lactate or histone level.
Correlation between plasma histones and PCT in patients with sepsis
The ROC curve analysis of the current study didn’t show the importance of the PCT level within 24 hours after admission for predicting the mortality rate of patients with sepsis. Elevated PCT levels are caused not only by bacterial infections, but also surgery, trauma, cardio-pulmonary resuscitation, tumor, and autoimmune disorders. These increases in PCT caused by non-infectious factors are considered false-positive results, which are more likely in critical patients. Furthermore, the role of PCT in predicting the prognosis of patients with sepsis is controversial (27-30).
Predictive value of plasma histones and other indicators for mortality of patients with sepsis
This study investigated the predictive value of indicators of mortality in septic patients in the ICU. The results showed that the sensitivity and specificity of either plasma histone level or SOFA score were highly predictive of mortality. Furthermore, the predictive effect of histones was more substantial than the SOFA score. The results also showed that TnI, NT-proBNP, blood lactate, and PCT had little significance in prognostic evaluations.
The most common scoring systems to evaluate the severity of critically ill patients are the APACHE II and SOFA. A higher score indicates a worse outcome (31,32). The present study found a good correlation between the serum histone level and SOFA score. The patients with a higher histone level had significantly higher SOFA score, cTnI, NT-proBNP, lactate, and PCT, and were more likely to receive vasopressor medication and suffer ICU mortality. These indicators are often used to estimate the severity and prognosis of diseases. Our results suggest that plasma histone is associated with severity and prognosis. The molecular mechanism of H4 in sepsis was not well known. Many studies indicate that histones can be released into the extracellular space in response to inflammatory challenge (5,8). The extracellular histones as DAMP proteins can drive immune activation and cytotoxicity through interaction with toll-like receptors (TLRs), complement and cell membranes phospholipids (33). In vitro, exogenous histones transient increase intracellular calcium concentration in endothelial cells; in vivo, histone administration causes neutrophil migration, endothelial injury and dysfunction, hemorrhage, and thrombosis (5).
In the current study, our patients had a mortality rate of 29.3% (37 deaths in 126 ICU patients). This is consistent with the 29% to 50% reported elsewhere (34-36). The SOFA score, plasma histone H4, and cTnI levels of the patients who died were significantly higher than the patients who survived. Furthermore, a higher histone H4 level correlated with a worse disease outcome. Therefore, we propose that plasma histones may be a useful biomarker for predicting outcome in patients with sepsis.
Based on the evidence of animal experiment, the infusion of histone antibodies may be a promising strategy in sepsis treatment for human beings. Future studies should investigate the prognostic value of a new sepsis assessment tool that would incorporate the plasma histone level into the current SOFA scoring system.
Coming to definite conclusions in this study is difficult. This study was conducted at one medical center, with a small number of cases. It should also be noted that the time intervals for the studies of each patient were 24 hours from the beginning of their ICU admission. Therefore, it is not known whether plasma histone levels can predict patient prognosis in the long term. Also, mortality associated with non-effective initial empirical treatment was not investigated, nor whether routine treatments (e.g., antibiotics, fluid resuscitation, vasopressors, or ventilator support) may influence the histone level. Future studies should address these issues.
Conclusions
In conclusion, a high plasma histone H4 level was a risk factor for an increased mortality rate in septic patients. Plasma histone H4 should be considered as a useful biomarker for evaluating the prognosis of sepsis.
Acknowledgments
Funding: This work was supported by the Ministry of Science and Technology of the People’s Republic of China (grant number 2012BAI11B05) and Beijing Municipal Science and Technology Commission (BSTC) (grant number D101100050010058).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1011
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1011
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1011). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Hospital Ethics Committees and was performed in full compliance with the Declaration of Helsinki. All the study participants, or their health care proxies, supplied informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shahreyar M, Fahhoum R, Akinseye O, et al. Severe sepsis and cardiac arrhythmias. Ann Transl Med 2018;6:6. [Crossref] [PubMed]
- Holdenrieder S, Stieber P, Bodenmuller H, et al. Nucleosomes in serum as a marker for cell death. Clin Chem Lab Med 2001;39:596-605. [Crossref] [PubMed]
- van Nieuwenhuijze AE, van Lopik T, Smeenk RJ, et al. Time between onset of apoptosis and release of nucleosomes from apoptotic cells: putative implications for systemic lupus erythematosus. Ann Rheum Dis 2003;62:10-4. [Crossref] [PubMed]
- Holdenrieder S, Stieber P. Clinical use of circulating nucleosomes. Crit Rev Clin Lab Sci 2009;46:1-24. [Crossref] [PubMed]
- Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med 2009;15:1318-21. [Crossref] [PubMed]
- Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood 2011;118:3708-14. [Crossref] [PubMed]
- Alhamdi Y, Zi M, Abrams ST, et al. Circulating Histone Concentrations Differentially Affect the Predominance of Left or Right Ventricular Dysfunction in Critical Illness. Crit Care Med 2016;44:e278-88. [Crossref] [PubMed]
- Kalbitz M, Grailer JJ, Fattahi F, et al. Role of extracellular histones in the cardiomyopathy of sepsis. FASEB J 2015;29:2185-93. [Crossref] [PubMed]
- Alhamdi Y, Abrams ST, Cheng Z, et al. Circulating Histones Are Major Mediators of Cardiac Injury in Patients With Sepsis. Crit Care Med 2015;43:2094-103. [Crossref] [PubMed]
- Gao H, Zhang N, Lu F, et al. Circulating histones for predicting prognosis after cardiac surgery: a prospective study. Interact Cardiovasc Thorac Surg 2016;23:681-7. [Crossref] [PubMed]
- Ekaney ML, Otto GP, Sossdorf M, et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit Care 2014;18:543. [Crossref] [PubMed]
- García-Giméne JL, Roma-Mateo C, Carbonell N, et al. A new mass spectrometry-based method for the quantification of histones in plasma from septic shock patients. Sci Rep 2017;7:10643. [Crossref] [PubMed]
- Scheer CS, Kuhn SO, Fuchs C, et al. Do Sepsis-3 Criteria Facilitate Earlier Recognition of Sepsis and Septic Shock? A Retrospective Cohort Study. Shock 2019;51:306-11. [Crossref] [PubMed]
- Barochia AV, Cui X, Eichacker PQ. The Surviving Sepsis Campaign’s Revised Sepsis Bundles. Curr Infect Dis Rep 2013;15:385-93. [Crossref] [PubMed]
- Zeerleder S, Stephan F, Emonts M, et al. Circulating nucleosomes and severity of illness in children suffering from meningococcal sepsis treated with protein C. Crit Care Med 2012;40:3224-9. [Crossref] [PubMed]
- Chen Q, Ye L, Jin Y, et al. Circulating nucleosomes as a predictor of sepsis and organ dysfunction in critically ill patients. Int J Infect Dis 2012;16:e558-64. [Crossref] [PubMed]
- Favory R, Neviere R. Significance and interpretation of elevated troponin in septic patients. Crit Care 2006;10:224. [Crossref] [PubMed]
- Yang Z, Qdaisat A, Hu Z, et al. Cardiac Troponin Is a Predictor of Septic Shock Mortality in Cancer Patients in an Emergency Department: A Retrospective Cohort Study. PLoS One 2016;11:e0153492. [Crossref] [PubMed]
- Wang J, Ji W, Xu Z, et al. Clinical significance of plasma levels of brain natriuretic peptide and cardiac troponin T in patients with sepsis. Exp Ther Med 2016;11:154-6. [Crossref] [PubMed]
- Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol 2009;30:513-21. [Crossref] [PubMed]
- Charpentier J, Luyt CE, Fulla Y, et al. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med 2004;32:660-5. [Crossref] [PubMed]
- Casserly B, Phillips GS, Schorr C, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med 2015;43:567-73. [Crossref] [PubMed]
- Frenzen FS, Kutschan U, Meiswinkel N, et al. Admission lactate predicts poor prognosis independently of the CRB/CURB-65 scores in community-acquired pneumonia. Clin Microbiol Infect 2018;24:306.e1-e6. [Crossref] [PubMed]
- Kang HE, Park DW. Lactate as a Biomarker for Sepsis Prognosis? Infect Chemother 2016;48:252-3. [Crossref] [PubMed]
- Bolvardi E, Malmir J, Reihani H, et al. The Role of Lactate Clearance as a Predictor of Organ Dysfunction and Mortality in Patients with Severe Sepsis. Mater Sociomed 2016;28:57-60. [Crossref] [PubMed]
- Gu WJ, Zhang Z, Bakker J. Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med 2015;41:1862-3. [Crossref] [PubMed]
- Huang DT, Weissfeld LA, Kellum JA, et al. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med 2008;52:48-58 e2.
- Garnacho-Montero J, Huici-Moreno MJ, Gutierrez-Pizarraya A, et al. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit Care 2014;18:R116. [Crossref] [PubMed]
- Karlsson S, Heikkinen M, Pettila V, et al. Predictive value of procalcitonin decrease in patients with severe sepsis: a prospective observational study. Crit Care 2010;14:R205. [Crossref] [PubMed]
- Jain S, Sinha S, Sharma SK, et al. Procalcitonin as a prognostic marker for sepsis: a prospective observational study. BMC Res Notes 2014;7:458. [Crossref] [PubMed]
- Naqvi IH, Mahmood K, Ziaullaha S, et al. Better prognostic marker in ICU - APACHE II, SOFA or SAP II! Pak J Med Sci 2016;32:1146-51. [Crossref] [PubMed]
- Choi JY, Jang JH, Lim YS, et al. Performance on the APACHE II, SAPS II, SOFA and the OHCA score of post-cardiac arrest patients treated with therapeutic hypothermia. PLoS One 2018;13:e0196197. [Crossref] [PubMed]
- Silk E, Zhao H, Weng H, et al. The role of extracellular histone in organ injury. Cell Death Dis 2017;8:e2812. [Crossref] [PubMed]
- Perner A, Cecconi M, Cronhjort M, et al. Expert statement for the management of hypovolemia in sepsis. Intensive Care Med 2018;44:791-8. [Crossref] [PubMed]
- Prescott HC, Angus DC. Postsepsis Morbidity. JAMA 2018;319:91. [Crossref] [PubMed]
- Reinhart K, Daniels R, Kissoon N, et al. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N Engl J Med 2017;377:414-7. [Crossref] [PubMed]


