
Geniposide attenuates dextran sulfate sodium-induced colitis in mice via Nrf-2/HO-1/NF-κB pathway
Introduction
Ulcerative colitis (UC) is a chronic nonspecific inflammatory disease. The pathological changes occur primarily in the colonic mucosa. Colonic mucosal inflammation and the formation of ulcers are the principal clinical and pathological features of UC. The UC lesions are concentrated mainly in the rectum and sigmoid colon, and the whole colon in severe cases. UC is characterized by clinical manifestations such as diarrhea, abdominal pain, mucus pus, bloody stools, and other symptoms (1). Immune abnormalities, genetic susceptibility, and intestinal microbial function are the principal pathogenic factors in UC (2). Various mediators, including cytokines, growth factors, adhesion molecules, and others play a vital role in the abnormal immune response. Current research on UC pathogenesis is focused on cytokines and inflammatory mediators responsible for the immune response and inflammatory processes in UC (3). The etiology and pathogenesis of UC are still unclear to date. Studies have shown the role of oxidative stress in UC development. Nuclear factor E2 related factor 2 (Nrf-2) plays a crucial role in oxidative stress processes. External injury factors can activate Nrf-2 and transfer it to stone, thus initiating transcription of various antioxidant enzymes to enhance the antioxidant capacity of local tissues, and help reduce the extent of oxidative stress response induced by external injury factors (4). To clarify the role of NRF-2 mediated oxidative stress in the occurrence and changes of UC.
Currently, although several methods exist for UC treatment, there is no specific cure, and the World Health Organization has ranked UC among the modern refractory diseases. Some drugs currently used for UC treatment lack specificity. Western medical treatment includes several drug categories, such as salicylic acid, hormones, immunosuppressants, antibiotics, biological agents, and others. Most of these could alleviate UC symptoms; however, they do not affect the natural course of the disease. Many Western medicine treatments exhibit varying therapeutic efficacy due to differences in individual drug tolerance, and the different side effects of drug treatment also limit drug use. Traditional medicine has a long history of UC treatment. Identification of new effective drugs from traditional Chinese medicine preparations might offer opportunities for UC treatment. A study on the treatment of UC with the traditional Chinese medicine preparations would help unravel the pathogenesis of UC and might provide opportunities for identifying highly effective drugs to treat UC.
Geniposide (GE) is a major component of the Gardenia jasminoides Ellis (Gardenia fruits) fruit, proved experimentally to possess multiple pharmacological activities, such as anti-inflammatory, anti-apoptotic, anti-oxidative stress, anti-angiogenic, and anti-ER stress (5). GE ameliorates dextran sulfate sodium (DSS)-induced colitis in mice (6). Although it showed the effect of GE on enteritis, its mechanism is relatively simple. In this study, we determined the therapeutic activity of GE in DSS-induced inflammatory bowel disease model mice.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-279).
Methods
Drugs and reagents
GE (purity: 97%) was purchased from Tianjin Wanxiang Hengyuan Technology Co., Ltd. Interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) ELISA kits were purchased from Elabscience (lot number: Ex 210804). All antibodies were purchased from the Cell Signaling Technology (Danvers, USA), and complete Freund’s adjuvant was from Sigma-Aldrich.
Animals
The experimental animal ethics committee approved all procedures of The Third Affiliated Hospital of Henan University of Traditional Chinese Medicine and consistent with the ethical guidelines for the investigation of experimental pain in animals (No. TAHHUTCM2018-0711). Thirty-six male ICR mice (8–12 weeks old) were obtained from Jiangning Qinglongshan Animal Cultivation Farm (Nanjing, China), and caged in special steel containers with ad libitum access to water and food. During the study, mice were kept in an air-conditioned room at 23±2 °C with a 12-h light/dark cycle.
Establishment of DSS-induced murine colitis model and treatment
Acute colitis was induced by the addition of DSS (MP Biomedicals, USA) to drinking water. Mice received either distilled water (control) or 3% (w/v) DSS in distilled water (experimental) continuously for 7 days, followed by distilled water for the next 3 days. Mice were assigned randomly to normal, DSS-treated, and GE (20 and 40 mg/kg)-treated groups. GE was administered by oral gavage from days 1–10.
Cell culture
RAW 264.7 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA, and cultured in Dulbecco’s Modified Eagle Media (DMEM) containing 5.5 mmol/L of D-glucose, and 10% fetal bovine serum. The cells were cultured at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
MTT assessment
The MTT assay was used to assess cell viability. RAW 264.7 cells were plated in 96-well plates at a density of 5,000 cells/well. The specific experimental steps were described previously. Cell viability was expressed as a percentage of vehicle-treated cells (control).
Nrf2 silencing by siRNA
RAW 264.7 cells were inoculated in 6-well culture plates and allowed to grow to 70% confluence. The Lipofectamine™ 2000 (Invitrogen Ltd., Carlsbad, CA) transfection reagent was used for transient transfection following the manufacturer’s instructions. Specific siRNA for Nrf2 isoforms, a (GGGUAAGUCGAGAAGUGUUTT), and b (AACACUUCUCGACUUACCCTT), and scrambled siRNA control were designed by Gene Pharma Co. (Shanghai, China). The reaction mixture containing 5 µL siRNA, 5 µL Lipofectamine™ 2000, and 95 µL serum-free culture medium (Opti-MEM, Invitrogen) was mixed at room temperature, and incubated for 20 min. Later, 800 µL of Opti-MEM medium was added drop-wise to each culture well containing the RAW 264.7 cells, and the reaction mixture added. After transfection for 6 h, the cell culture medium was replaced, and cells incubated for another 24 h before exposure to lipopolysaccharide (LPS). The knockdown efficiency was validated by western blotting analysis.
Evaluation of myeloperoxidase (MPO) activity in the colon
MPO activity was determined following an established protocol (7).
ROS detection
For ROS detection, RAW 264.7 cells or and Nrf2 siRNA treated RAW 264.7 cells were pre-treated with GE for 24 h, the cells were followed by washing with serum-free medium, and incubated with (2,7-Dichlorodihydrofluorescein diacetate) DCFH-DA (10 µM) for 20 min at 37 °C. The fluorescence intensity was measured at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Estimation of superoxide dismutase (SOD) and malondialdehyde (MDA) levels in the serum, colon, and cell supernatant
The SOD and MDA content in the serum and colon of mice in each group and the cell supernatants were estimated following the kit instructions.
Determination of cytokines in serum, colon, and cell supernatant
The IL-1β, IL-6, and TNF-α content in serum, colon, and cell supernatant were measured following the instructions of ELISA kits.
Pathological examination of the colon
Immediately after the mice were euthanized, the colon was removed and fixed in 10% formalin dragon solution. The intestinal tissue was dehydrated, processed in xylene, paraffin-embedded, and sectioned. The sections were stained with hematoxylin-eosin (H&E) stain, and the pathological changes observed under a microscope (Nikon, Tokyo, Japan) at 20× magnification.
Western blotting analysis
The protein expression in the colon tissue and RAW 264.7 cells was evaluated by western blotting. Myocardial tissue and cells were homogenized in ice-cold radioimmunoprecipitation assay (RIPA) buffer with 0.1% benzenesulfonyl fluoride. The lysates were centrifuged at 12,000 ×g for 20 min, and the protein concentration determined with Coomassie-blue reagent. Subsequently, protein extracts from different groups were separated on SDS-polyacrylamide gel electrophoresis and transferred to the polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was blocked with 5% skim milk at room temperature for 2 h and incubated with the respective primary antibodies overnight at 4 °C. After washing with Tris Buffered Saline Tween (TBST) three times, the PVDF membrane was incubated with the HRP-conjugated secondary antibodies. Subsequently, the developed bands visualized by enhanced chemiluminescence (ECL) advanced kit and gel imaging system (China Tanon Technology Co., Ltd.).
Statistical analysis
GraphPad Prism version 7.0 (GraphPad Software Inc., San Diego, CA, USA) was used for data analysis. One-way ANOVA was used for comparisons among different groups. The values are presented as mean ± SD. P<0.05 indicated statistical significance.
Results
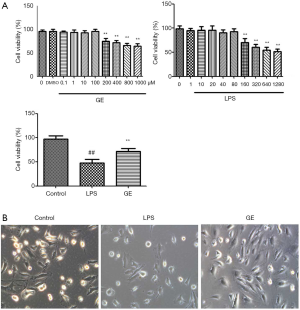
GE protects cells against LPS-induced cytotoxicity
The effect of GE on RAW 264.7 cell viability was assessed via MTT assay. Incubation of cells with 0.1–100 µM GE for 24 h did not affect RAW 264.7 cell viability, while incubation with 200–1,000 µM GE markedly impaired RAW 264.7 cell viability.
Next, we examined the protective effect of GE on LPS-induced cellular damage in RAW 264.7 cells. Figure 1 shows that incubation with LPS reduced the cell viability significantly, and GE pretreatment restored the cell viability considerably.
The effect of GE on ROS in LPS-induced RAW 264.7 cells
As shown in Figure 2, the ROS levels in LPS-induced RAW 264.7 cells increased, and GE (1,000 µM) decreased the ROS levels in RAW 264.7 cells. However, the treatment of RAW 264.7 cells with Nrf-2 siRNA eliminated the effect of GE on ROS levels.

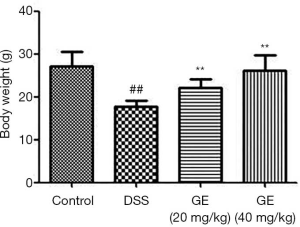
The effect of GE on body weight in mice
Figure 3 shows significantly decreased body weight in DSS-induced mice, compared to control mice, and GE (20 and 40 mg/kg) increased body weight in DSS-induced mice markedly.
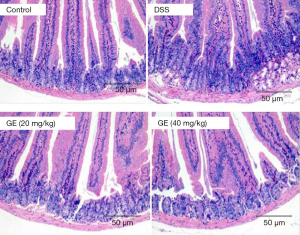
The effect of GE on pathological changes
Histological analysis showed erosion and distortion of crypts, loss of glandular epithelium, and inflammatory cell infiltration in DSS-treated mice. In the experimental group, we observed significant improvement in colon pathology in mice treated with GE (Figure 4).
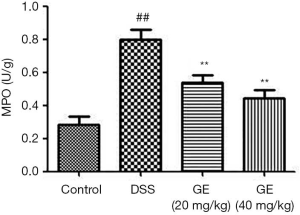
The effect of GE on MPO in the colon
Figure 5 shows significantly higher MPO levels in DSS-induced mice compared to control mice, and the administration of GE (20 and 40 mg/kg) decreased MPO levels dramatically.
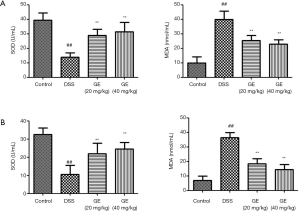
GE reduces colonic MDA content and enhances SOD activity levels in serum and colon
Oxidative stress in the intestinal tissues leads to the production of increased oxidative stress by-products in the blood. Hence, it is critical to monitor intestinal oxidative stress products in the serum. Figure 6 shows increased MDA content and decreased SOD activity levels in the serum and colon of DDS-induced mice compared to control mice, and the administration of GE (20 and 40 mg/kg) reversed the effects on MDA and SOD.
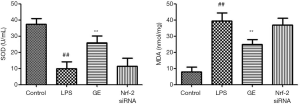
The effect of GE on MDA content and SOD activity levels in the cell supernatants
LPS treatment increased MDA and decreased SOD levels in RAW 264.7 cells (Figure 7), and as expected, pretreatment with GE (1,000 µM) increased SOD and decreased the MDA level, and Nrf-2 siRNA treatment of cells blocked this effect.
The effect of GE on inflammatory cytokines in serum and colon
Figure 8 shows increased IL-1β, IL-6, and TNF-α levels in serum and colon of DSS-induced mice, and GE (20 and 40 mg/kg) treatment decreased IL-1β, IL-6, and TNF-α levels in serum and colon significantly.

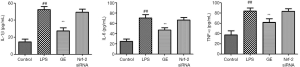
The effect of GE on inflammatory cytokines in the cell supernatant
LPS increased IL-1β, IL-6, and TNF-α levels in RAW 264.7 cells (Figure 9). Treatment with GE (1,000 µM) decreased the levels of IL-1β, IL-6, and TNF-α significantly, and Nrf-2 siRNA reversed this effect.
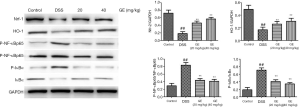
The effect of GE on Nrf-2/HO-1/NF-κB pathway in DSS-induced mice
Figure 10 shows decreased levels of Nrf-2 and HO-1 and increased p-NF-κBp65 and p-IκBα levels in the colon of DSS-induced mice. Administration of GE (20 and 40 mg/kg) increased the levels of Nrf-2 and HO-1 and decreased p-NF-κBp65 and p-IκBα levels significantly.
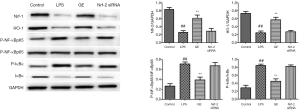
The effect of GE on Nrf-2/HO-1/NF-κB pathway in LPS-induced RAW 264.7 cells
The Nrf-2 and HO-1 levels decreased while p-NF-κBp65 and p-IκBα levels increased in LPS-induced RAW 264.7 cells (Figure 11), and GE (1,000 µM) increased Nrf-2 and HO-1 levels and decreased the levels of p-NF-κBp65 and p-IκBα significantly.
Discussion
In recent years, significant progress has been made in UC clinical and basic research. It is particularly important to establish a stable, reliable, simple, and reproducible animal model to understand the etiology and pathogenesis of the disease further and for evaluating the efficacy of therapeutic drugs. Currently, it is believed that the model for acute UC deduced by the decision support system mimics closely the pathological changes observed in intestinal inflammation during the acute phase of human UC (8). The mechanism of this method may be that the decision support system inhibits the proliferation of intestinal epithelial cells and damages intestinal mucosal barrier function, resulting in intestinal flora imbalance and immune dysfunction (9).
DSS is a sulfated polysaccharide, synthesized from sucrose and possesses anti-hemorrhagic and anti-coagulative functions similar to heparin. In 1986, researchers successfully established a UC animal model using a decision support system and showed that the pathological changes observed in the UC animal model mimic human UC, rather than other animal models (10). Since then, UC experimental studies are focused widely on developing animal models with decision support systems. According to relevant literature reports, DSS in drinking water was used to establish the mouse model, and mice received 5% DSS in water for 1 week to develop an acute UC animal model (11,12). During this period, DSS-induced mice showed significant weight loss based on their activity and weight measurements. The stool occult blood test showed a positive reaction. Microscopically, thin blood adhered to anus, listlessness, laziness, curled up into a ball, panicky, messy lusterless fur, hyperemia and edema of the colon, and hemorrhage and ulcer in severe cases. A large number of inflammatory cells infiltrated the mucosa and submucosa.
Nrf2 is an exogenous poison and oxidative stress receptor and is an important transcription factor for maintaining the cellular redox balance (13). Therefore, it plays a vital role in cellular oxidative stress processes. Meanwhile, HO-1 is the target gene of the Nrf2/HO-1 signaling pathway, and Nrf induces upregulation of HO-1 expression, inhibits the production and release of inflammatory mediators, and acts as an anti-inflammatory, antioxidant, and anti-apoptotic agent to protect cells (14,15). The Nrf2/HO-1 signaling pathway is involved in the pathogenesis of many diseases. The upregulated Nrf2/HO-1 signaling pathway might have an anti-inflammatory role in alleviating ischemia-reperfusion injury and cellular protection (16,17). In this study, the Nrf2/HO-1 pathway was inhibited in DSS-induced mice and upregulated in the Nrf-2 siRNA group. The experimental results showed that the Nrf2/HO-1 signaling pathway participates in the pathogenesis of IBD.
NF-κB was first discovered and reported by Sen et al. in 1995, identified and extracted from lymphocyte nuclear extract (18). NF-κB is a regulatory factor that specifically binds to the B-lymphocyte immunoglobulin-K light-chain gene enhancer sequence and has transcriptional regulatory activity. NF-κB is a multidirectional transcription regulatory nucleoprotein factor, which participates in the pathogenesis of many inflammatory diseases (19). NF-κB not only regulates the activation and expression of several cytokines but also acts as an important regulator of various inflammatory and immune response-related factors. Some studies have shown a crucial role for NF-κB in regulating inflammatory cell, apoptotic, and immune regulatory gene expression (20-22). Later research identified that any gene containing the κB site could regulate transcription through NF-κB, including the association of several genes with immunity, inflammation, and transcription regulation, which might affect many physiological and pathological processes such as apoptosis, tumor cell adhesion, and metastasis. The genes containing κB sites include various cytokines and their receptors. Several factors could activate NF-κB during disease state or pathological processes. NF-κB nuclear translocation occurs after activation, thus promoting the secretion of a large number of inflammatory cytokines. NF-κB can also be activated further by these cytokines, aggravating intestinal immune response and inflammatory injury in patients with UC. The activation and expression of NF-κB might be one of the critical steps in UC development. NF-κB P65 is one of the five members of the NF-κB family and is a vital pro-inflammatory factor in IBD. In this study, the NF-κB pathway is activated in the DSS-induced mice group and downregulated in the Nrf-2 siRNA and mesalazine groups. The experimental results showed that the NF-κB signaling pathway participates in the pathogenesis of IBD.
In conclusion, our findings showed that GE protects mice from DSS-induced colitis through activation of the Nrf2/HO-1 signaling pathway for enhancing the antioxidant capacity and inhibiting the pro-inflammatory mediators in the colon. In addition, our research provides evidence that Nrf-2 siRNA targeting regulates the NF-κB pathway and alleviates DSS-induced colitis in mice and LPS-induced RAW 264.7 cells. However, our research is preliminary, and further studies are warranted.
Acknowledgments
Funding: This work was supported by Jiangsu Youth Medical Talents Project (QNRC2016255); Scientific and Technological Project of Henan Province (182102311146), National Training Program for Innovative Backbone Talents of Traditional Chinese Medicine {Chinese medicine renjiaohan [2019] No.128}.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-279
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-279
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-279). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The experimental animal ethics committee approved all procedures of The Third Affiliated Hospital of Henan University of Traditional Chinese Medicine and consistent with the ethical guidelines for the investigation of experimental pain in animals (No. TAHHUTCM2018-0711).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cross R, Ko CW, Singh S. Mild-to-Moderate Ulcerative Colitis Guideline. Gastroenterology 2019;156:768. [Crossref] [PubMed]
- Wu T, Dai Y, Xue L, et al. Expression of receptor interacting protein 3 and mixed lineage kinase domain-like protein-key proteins in necroptosis is upregulated in ulcerative colitis. Ann Palliat Med 2019;8:483-9. [Crossref] [PubMed]
- Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol 2018;30:1-10. [Crossref] [PubMed]
- Yuan Z, Yang L, Zhang X, et al. Huang-Lian-Jie-Du Decoction Ameliorates Acute Ulcerative Colitis in Mice via Regulating NF-κB and Nrf2 Signaling Pathways and Enhancing Intestinal Barrier Function. Front Pharmacol 2019;10:1354. [Crossref] [PubMed]
- Zhou YX, Zhang RQ, Rahman K, et al. Diverse Pharmacological Activities and Potential Medicinal Benefits of Geniposide. Evid Based Complement Alternat Med 2019;2019:4925682. [Crossref] [PubMed]
- Zhang Z, Li Y, Shen P, et al. Administration of geniposide ameliorates dextran sulfate sodium-induced colitis in mice via inhibition of inflammation and mucosal damage. Int Immunopharmacol 2017;49:168-77. [Crossref] [PubMed]
- Smith JW, Castro GA. Relation of peroxidase activity in gut mucosa to inflammation. Am J Physiol 1978;234:R72-9. [PubMed]
- Liu L, Liu Y, Cui J, et al. Oxidative stress induces gastric submucosal arteriolar dysfunction in the elderly. World J Gastroenterol 2013;19:9439-46. [Crossref] [PubMed]
- Brandtzaeg P. Homeostatic impact of indigenous microbiota and secretory immunity. Benef Microbes 2010;1:211-27. [Crossref] [PubMed]
- Sanderson IR. Chronic inflammatory bowel disease. Clin Gastroenterol 1986;15:71-87. [PubMed]
- Zhu H, Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med (Maywood) 2012;237:474-80. [Crossref] [PubMed]
- Wang A, Keita ÅV, Phan V, et al. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am J Pathol 2014;184:2516-27. [Crossref] [PubMed]
- Bellezza I, Giambanco I, Minelli A, et al. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res 2018;1865:721-33. [Crossref] [PubMed]
- Zeng T, Zhang CL, Song FY, et al. The activation of HO-1/Nrf-2 contributes to the protective effects of diallyl disulfide (DADS) against ethanol-induced oxidative stress. Biochim Biophys Acta 2013;1830:4848-59. [Crossref] [PubMed]
- Park EJ, Kim YM, Park SW, et al. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem Toxicol 2013;55:386-95. [Crossref] [PubMed]
- Liu C, Zhu C, Wang G, et al. Higenamine regulates Nrf2-HO-1-Hmgb1 axis and attenuates intestinal ischemia-reperfusion injury in mice. Inflamm Res 2015;64:395-403. [Crossref] [PubMed]
- Wang J, Hu X, Xie J, et al. Beta-1-adrenergic receptors mediate Nrf2-HO-1-HMGB1 axis regulation to attenuate hypoxia/reoxygenation-induced cardiomyocytes injury in vitro. Cell Physiol Biochem 2015;35:767-77. [Crossref] [PubMed]
- Sen J, Venkataraman L, Shinkai Y, et al. Expression and induction of nuclear factor-kappa B-related proteins in thymocytes. J Immunol 1995;154:3213-21. [PubMed]
- Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol 2017;17:545-58. [Crossref] [PubMed]
- Mitchell JP, Carmody RJ. NF-κB and the Transcriptional Control of Inflammation. Int Rev Cell Mol Biol 2018;335:41-84. [Crossref] [PubMed]
- Timucin AC, Basaga H. Pro-apoptotic effects of lipid oxidation products: HNE at the crossroads of NF-κB pathway and anti-apoptotic Bcl-2. Free Radic Biol Med 2017;111:209-18. [Crossref] [PubMed]
- Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res 2011;21:223-44. [Crossref] [PubMed]


