The role of hypofractionated radiation in the management of non-osseous metastatic or uncontrolled local cancer
Editor’s note:
“Palliative Radiotherapy Column” features in articles emphasising the critical role of radiotherapy in palliative care. Chairs to the columns are Dr. Edward L.W. Chow from Odette Cancer Centre, Sunnybrook Health Sciences Centre in Toronto and Dr. Stephen Lutz from Blanchard Valley Regional Cancer Center in Findlay, gathering a group of promising researchers in the field to make it an excellent column. The column includes original research manuscripts and timely review articles relating to palliative radiotherapy, and editorials and commentaries on recently published trials and studies as well.
Introduction
Radiation therapy is often used to palliate symptoms caused by cancer. In this article, the authors will review the literature supporting short course, or “hypofractionated” palliative radiation therapy in the setting of non-osseous metastasis or uncontrolled localized cancer. A recent review summarizes the literature surrounding the use of radiotherapy (RT) in the palliation of osseous metastasis (1).
Hypofractionated palliative radiation plays a role in the management of obstruction due to tumor, neurologic symptoms, pain caused by localized bulky disease, and bleeding in patients with primary tumors of the lung, head and neck, bladder, rectum, gynecologic malignancies, and metastasis to the liver, lung and brain. It may provide equivalent palliation and relief while being time-efficient, cost effective and less toxic than longer courses of radiation therapy.
Hypofractionated radiation therapy
The earliest uses of radiation therapy employed a single treatment (or a small number of fractions) of large doses of radiation. Treatment was often limited by excessive skin reactions without meaningful effect on the underlying malignancy. Through experimentation, treatment evolved to a larger number of smaller doses (or fractions) of radiation delivered 5 days per week over several weeks. These smaller doses are on the order of 1.8-2 Gy per fraction, for a period of 5-8 weeks. The exact treatment schedule is based on the clinical circumstances and tumor histology (2).
Hypofractionated radiation therapy, or the use of a smaller number of larger size fractions (3-8 Gy) of radiation, is ideally suited for palliation of symptoms due to metastatic cancer. Treatment courses are shortened, which is important when the quantity of days remaining is short and quality of life (QOL) is the primary goal of treatment. Patients in this phase of life tend to be uncomfortable with transport. For these patients, the potential for increased risk of long-term side effects that are associated with larger doses per fraction related to late-responding tissues is less relevant. In addition, the lower biologic equivalent dose of these shorter courses can further minimize this risk of late side effects.
Many of the studies of hypofractionated radiation therapy in the palliation of uncontrolled local disease or metastatic cancer are retrospective, single-institution reviews that were performed in an era prior to widespread use of QOL questionnaires and metrics (3). The most robust data on the equivalence in efficacy, increased convenience, lower cost and less toxicity resides in the setting of single fraction RT for osseous metastasis. The same principles of efficacy, convenience, cost and toxicity apply to hypofractionated radiation therapy for treatment of non-osseous metastasis that is the focus of this review.
The perceived reluctance of radiation oncologists to offer short course palliation is cited by palliative care professionals as a reason that patients on hospice do not often get referred for palliative radiation, though those same physicians recognize the palliative benefit that radiation can provide for conditions such as painful bone metastases (4). Those same clinicians are less familiar with the potential benefits of palliative RT for tumor-related symptoms beyond painful bone metastases (4,5). Palliation of symptoms such as hemoptysis and bleeding due to tumors in various locations, obstruction of airways or other organs, pain or neurologic symptoms caused by local tumor growth and brain metastasis is the subject of this review. Key to the administration of any palliative radiation is the expectation that a patient will live long enough to see a benefit from palliative radiation. Palliative effects of radiation can be seen within 24-48 hours for bleeding but can take several weeks for the full palliative effect in other settings.
Lung cancer/palliation of disease in the lung
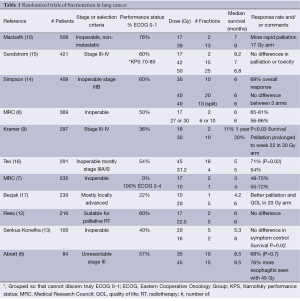
Eleven randomized trials and one non-randomized trial have compared palliative radiation therapy schemes for patients with lung cancer (6-17) (Table 1). In addition, six single-arm prospective trials (18-23) and multiple retrospective reviews have looked at hypofractionated radiation therapy for this patient group. The vast majority of these studies were conducted outside the US without the routine use of chemotherapy. No consistent mechanism for scoring symptoms, QOL or degree of palliation was used.
Full table
Fractionation regimens
Seven of these trials compared a 2-fraction regimen with longer (5-25 fractions) courses of radiation therapy. The vast majority of these trials did not demonstrate any benefit to the longer courses of radiation therapy, with a few notable exceptions.
Two trials (9,10) did suggest a survival advantage to the longer courses of radiation, while one (13) demonstrated a survival benefit to the shorter course regimen. One study (10) noted shorter duration of esophagitis with the 2-fraction regimen while another (12) demonstrated a trend toward more esophagitis in the 2-fraction regimen. Two studies (10,12) noted better palliation with the 2-fraction regimens though one (9) demonstrated more prolonged palliation with 10 fractions.
Two trials (7,17) compared a single fraction of 10 Gy to either 2- or 5-fraction courses in patients with poor performance status (PS). Whereas the Medical Research Council (MRC) trial did not demonstrate any benefit to the 2-fraction regimen as compared to a single fraction, the National Cancer Institute of Canada (NCIC) trial noted a 2-month improvement in survival, better symptom relief and improved QOL with 5 vs. 2 fractions. One explanation for this difference may be the proportion of patients with poor PS; in the NCIC trial 40% of patients were Eastern Cooperative Oncology Group (ECOG) 0-1 compared to 0% in the MRC trial. One theme that emerges throughout the palliative literature is that PS is a strong predictor of survival (24).
Performance status (PS)
Seven of the randomized trails had more than 50% of patients with ECOG 0-1 (6,8,10,12,14-16). Of these seven trials, only one (10) suggested a survival benefit for the longer course of radiation therapy. Macbeth et al. randomized 509 patients to either 17 Gy in 2 fractions or 39 Gy in 13 fractions. More rapid palliation and a shorter duration of radiation-induced esophagitis were seen in the 2-fraction regimen. There was a small survival advantage seen in the 13-fraction regimen with a hazard ration of 0.82 [95% confidence interval (CI): 0.69-0.99, P=0.03]. One large caveat with applying this trial to patients treated with palliative intent in the US is that patients with locally advanced, non-metastatic lung cancer were eligible. Though not specifically stated, this designation presumably correlates to stage IIIA and IIIB lung cancer, which in patients with ECOG 0-1, are typically treated curatively with concurrent chemoradiation in the US. Stage III lung cancer typically involves lymph nodes in the ipsilateral mediastinum (IIIA) or contralateral mediastinum, supraclavicular lymph nodes or multiple tumors in the same lung (IIIB). It remains to be seen whether the survival advantage would be seen in a similar population of stage IIIB/IV patients given that no significant differences in local recurrence rates were seen between the groups at 6, 12 or 24 months. Distant disease was seen as the site of first failure more commonly in those treated with the 2-fraction regimen.
In the five trials largely comprised of patients with ECOG ≥2, only one (9) demonstrated a survival advantage for the longer fraction course. Kramer et al. enrolled patients with ECOG 3-4/stage III disease as well as ECOG 0-1/Stage IV lung cancer. In a subgroup analysis, the improved survival seen with the longer course of radiation was seen only in the patients with ECOG 0-1.
In an effort to decrease travel time for patients at the end of life, Plataniotis et al. (11) treated 92 patients with either 17 Gy in 2 fractions given 1 week apart vs. 4.25 Gy given TID on day 1 and BID on day 2 with a 4-hour inter-fraction interval. Patients were not randomized but were assigned to a treatment group based upon the distance they would have to travel to receive treatment. There was no clinically significant difference in toxicity, efficacy, or survival between the two different fractionation schemes; however, the sample size was small.
Fairchild et al. (25) performed a systematic review of 13 randomized trials of fractionation in the palliation of symptomatic lung cancer. No significant differences were seen with respect to symptom control. Their review suggested an improvement in survival that favored fractionation regimens with higher biologic effective dose [biologically effective dose (BED) ≥35 Gy10]. One caveat to their conclusion is that only 28% of patients included in the analysis had extrathoracic metastatic disease, again leaving open the question of whether there truly is a survival benefit associated with a particular fractionation regimen for patients with metastatic disease.
American Society for Radiation Oncology (ASTRO)’s recent evidence-based clinical practice guideline on palliative thoracic RT (26) noted that despite a heterogeneity of data in 14 randomized controlled trials, there was a benefit to palliative radiation therapy without favoring a particular fractionation regimen. Toxicity was noted to be generally mild but increased with regimens that had a higher BED. No difference was found in response rates (RRs). There was a suggestion of survival benefit seen for longer fractionation regimens in some of the trials for those patients with a good PS at the expense of higher toxicity, cost and decreased convenience. It suggested that 2-fraction regimens are more easily integrated between cycles of systemic therapy, though there is limited data in this setting. Patients treated on the studies of palliative fractionation regimens did not get systemic therapy. Further research is needed to determine how best to integrate systemic therapy with palliative radiation regimens.
Risk for radiation myelitis
The MRC (27) analyzed the data from their three trials of hypofractionated radiation therapy and reported five cases of spinal cord injury consistent with radiation myelopathy. Three of 524 patients treated with 17 Gy/2 regimens and 2/153 patients treated with 39 Gy/13 were included on this list. None of the other radiation schemes had any myelitis noted. The 2-year cumulative rate of myelitis was approximately 2% for 17 Gy/2 and 39 Gy/13 and 0% for the other regimens. Symptoms began no earlier than 8 months after delivery of treatment, a time frame that is longer than most patients treated in this manner survive. Despite this being a rare complication, its severity can cause profound patient morbidity, and techniques to decrease the dose to the spinal cord, such as a posterior spinal cord block or an off-cord oblique beam, seem reasonable to minimize the risk of myelopathy.
Summary
The vast majority of trials of hypofractionated palliative radiation demonstrate equivalence in outcomes and toxicity for shorter courses of radiation therapy. These 2-fraction regimens can easily be integrated between cycles of systemic therapy. The increased convenience and decreased cost associated with 2-fraction regimens have established this regimen as an accepted standard of care for palliative lung radiation therapy in the setting of metastatic lung cancer that should be increasingly utilized in place of more protracted regimens.
Gynecologic (GYN), genitourinary (GU) or gastrointestinal (GI) cancer/palliation of disease in the pelvis
Uncontrolled tumor in the pelvis can cause bleeding, discharge, pain, obstruction or hydronephrosis. Despite the fact that patients with advanced pelvic malignancies have relatively short life-expectancy, these symptoms can be very problematic during their remaining life. Various hypofractionated radiation regimens have been utilized in an attempt to maximally palliate these patients while minimizing uncomfortable travel, cost and time in the hospital.
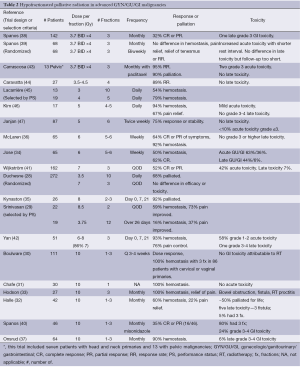
One randomized trial (28), one non-randomized comparison (29) and at least 12 single-arm studies (28-42) have been conducted (Table 2).
Full table
Among the earliest hypofractionated palliative schedules used to treat advanced pelvic malignancies was a regimen of 10 Gy delivered to the whole pelvis and repeated monthly for 1-3 fractions (30). Field reductions were utilized for the second and third fractions and megavoltage photon beams were used in the vast majority of patients. A dose response curve was noted ranging from 45% palliation of bleeding with a single fraction to 100% with 3 fractions in the patients with cervical or vaginal primaries. Similar dose responses were noted for other symptoms and disease regression. The vast majority of these patients did not achieve local control for their remaining short (median survival 3-9 months) lifespan. Serious bowel complications were all related to uncontrolled local tumor. No significant palliation was noted for those treated for endometrial or ovarian primary tumors. In contrast, in a study of patients with endometrial and cervical primaries treated with a single 10 Gy fraction (32), approximately 50% of patients were palliated with this regimen for their remaining lifetime. Five of 42 patients suffered late GI toxicity, namely fistula, developing 10 months or longer after the completion of radiation.
Several themes emerge from the series that have used 10 Gy for 1-3 fractions. First, it is extremely effective at controlling bleeding from pelvic malignancies. Secondly, acute toxicity is generally mild and time-limited. Lastly, there is an increased rate of late grade 3-4 GI toxicity, predominantly fistulae, that occurs beginning 9 months from the completion of treatment. Limiting the use of the third fraction, reducing the field size for subsequent fractions and selecting patients with shorter life expectancies have been suggested as means to decrease the rate of late GI toxicity. In addition, many of the series reported local recurrences within patients’ short remaining lifespan, though the short and incomplete follow-up in many of these series limits the ability to make firm conclusions about the duration of the palliative effect.
In response to the late GI toxicity seen in Radiation Therapy Oncology Group (RTOG) 79-05, a phase I trial of 10 Gy repeated monthly with misonidazole (40), the RTOG developed the “Quad Shot”. This regimen consists of 3.7 Gy given twice per day times 4 over 2 days repeated at monthly intervals times 3, if patients did not progress or did not decline clinically. In the initial trial, there was one late grade 3 toxicity and a 32% RR. A randomized trial (39) was undertaken to determine if the RR would increase if the interval between repetitions of the Quad Shot was shortened to 2 vs. 4 weeks. No difference in hemostasis, pain relief, relief of tenesmus or tumor response was noted between arms but the shorter interval was associated with increased acute toxicity. When combined with paclitaxel (43) in a small single arm study, RRs increased to 95% with 90% of patients palliated.
Rectal cancer
In patients with locally advanced and metastatic rectal cancer (47), a hypofractionated radiation therapy course can limit the need for palliative colostomy to 33%. This regimen is associated with an 82% local control rate with less than 10% mild acute toxicity and no late toxicity. For those with pain due to recurrent, unresectable rectal cancer, pain relief can be achieved in 70-90% of patients receiving palliative radiation therapy (48).
Bladder cancer
Hypofractionated regimens have been successfully employed in the treatment of elderly patients with bladder cancer who are considered incurable due to age, comorbidities and PS (28,29,34,36,45). One trial utilized a hypofractionated approach, but their inclusion criteria allowed for patients that were treated for cure and thus is not discussed in this article (49). Palliation of hematuria (50-79% of patients) and pain (52-76% of patients) can be achieved in patients with metastatic bladder cancer. RRs vary from 52-64% in the series where it is reported. Most studies note equivalence in regimens in terms of palliation, response and toxicity, though many also report acute worsening of urinary symptoms due to treatment (34,41). In two of the series (29,45) the fractionation scheme was based on PS with worse PS patients receiving more hypofractionated, shorter courses of radiation. In both of those series the more hypofractionated arms achieved better palliation of pain and hemostasis. Pain relief and hemostasis was achieved in 73% and 59% in the 2-fraction arm compared to 37% and 16% with the longer course of radiation in the series by Srinivasan (29). Nearly 80% of patients had recurrence of hematuria if they survived 6 months after treatment (45). However, the only randomized trial of fractionation in bladder cancer by Duchesne et al. (28) did not demonstrate any difference in survival, efficacy or toxicity between the shorter and longer courses of radiation. Sixty-eight percent of patients were effectively palliated. Palliation of hematuria, frequency, dysuria and nocturia was achieved in 88%, 82%, 72% and 64%, respectively. The two radiation regimens studied in this trial were similar to the non-randomized series.
Summary
Multiple alternative hypofractionated regimens have been studied in advanced pelvic malignancies with generally good palliative effects and reasonable toxicity. Many of these studies utilized 2-dimensional treatment; more conformal therapy should maintain the effective palliative response with decreased toxicity.
Head and neck cancer
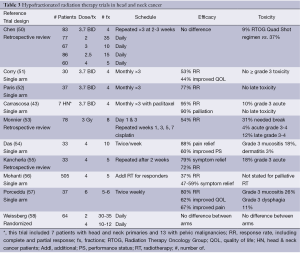
Locally advanced tumors of the head and neck pose significant local problems for patients during their remaining lifespan. Multiple prospective and retrospective studies have reported reasonable RRs and palliation with reasonable morbidity (Table 3).
Full table
Only one randomized controlled trial of fractionation has been performed in the setting of surgically unresectable stage III and IV head and neck cancer (58). Weissberg et al. did not demonstrate any significant difference in efficacy or toxicity between 60-70 Gy in 30-35 fractions when compared with 40-48 Gy in 10-12 fractions.
A phase II prospective study of 3.7 Gy BID times 4 over 2 days (the “Quad Shot”) was undertaken by Corry et al. (51). This Quad Shot was then repeated at monthly intervals times 3, if patients did not progress or decline clinically. The median survival was 5.7 months. No toxicity greater than or equal to grade 3 was noted. The tumor RR was 53% and 44% of patients experienced improvements in their QOL. The Quad Shot has been studied with concurrent paclitaxel (43) with an increase in the RR to 95% and 90% palliation of symptoms without any increase in late toxicity but with a 10% rate of acute grade 3 toxicity.
Chen et al. performed a retrospective review of five different fractionation regimens (50). No difference was seen in efficacy between the various regimens, but the RTOG Quad Shot regimen was associated with significantly less acute toxicity (9%) when compared to all of the other regimens (37%).
Mohanti et al. (56) studied patients with poor-prognosis, unresectable stage IV head and neck cancer. Patients were initially treated with a palliative regimen of 20 Gy in 5 fractions over 1 week. Those patients that had significant regression went on to receive conventionally fractionated radiation to the dose equivalent of 70 Gy2. Those who received full dose RT survived for a median of 13 months when compared to 6 months for non-responders. Overall the RR for those receiving palliative radiation was 37% with 47-59% palliation of symptoms.
Though multiple regimens have been studied in the palliative treatment of locally advanced head and neck cancer, the RTOG “Quad Shot” offers the best combination of efficacy, safety and patient convenience. This regimen has been associated with RRs of 53-95% and has been successfully combined with chemotherapy. The monthly interval between repeats allows non-responders and those whose PS declines to discontinue treatment.
Palliation of hepatic metastasis
Many of the studies of liver radiation for metastatic disease give whole liver irradiation plus or minus a boost (59,60). Given advances in chemotherapeutic agents, this approach has limited relevance. In an effort to improve efficacy and minimize the liver toxicity associated with whole liver radiation therapy, others have studied an accelerated hyperfractionated (more than 1 fraction per day at a dose lower than 1.8-2 Gy) approach (61). Palliative treatment of liver metastasis can be divided into purely palliative radiation (the scope of this article) vs. stereotactic body irradiation of oligometastatic disease, a discussion of which is beyond the scope of this article. Interested readers are directed to the following reference for further reading on that topic (62).
The Trans-Tasman Radiation Oncology Group looked at 2 fractions of 5 Gy given to the liver tumors plus a margin to relieve symptoms due to hepatic metastasis such as abdominal distention, hepatic pain, night sweats, nausea and/or vomiting. At 2 weeks, 50-66% improvement in individual symptoms was noted. Progressive relief of hepatic pain was noted through the 10 weeks follow-up period. There was a 7% grade 3 toxicity rate and 75% of patients perceived a benefit from treatment. A pain flare was noted in four patients (62,63).
Brain metastasis
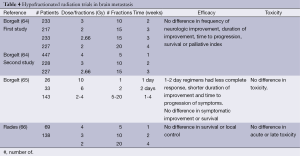
The most extensive studies of fractionation, comprising ~2,000 patients treated with RT for brain metastasis, were conducted by the RTOG (64,65). Two studies enrolled patients to one of five different radiation regimens (40 Gy in 20 fractions, 40 Gy in 15 fractions, 30 Gy in 15 fractions, 30 Gy in 10 fractions, or 20 Gy in 5 fractions). Selected institutions also participated in ultra-hypofractionated randomization consisting of 1-2 fractions of radiation (10 Gy in 1 fraction or 12 Gy in 2 fractions) (65). There was no difference in any of the five radiation regimens in terms of the frequency of symptom improvement, the duration of symptom improvement, the time to progression of disease, overall survival or the palliative index. Both the patient’s initial performance and neurologic status predicted response (64). Table 4 provides a summary of the trials of fractionation in the treatment of brain metastasis.
Full table
There was some concern that patients considered to have a more favorable prognosis may benefit from the higher dose radiation fractionation regimens and were evaluated in a subgroup analysis (67). Though there was a subgroup of breast cancer patients that had a significantly longer median survival (37 weeks compared to 11 weeks), there was no difference in the time to progression or death without progression of neurologic symptoms between the various fractionation regimens. The same held true for those with lung cancer and other/unknown primaries. No significant benefit was seen to longer radiation therapy courses.
In a retrospective review of breast cancer patients treated with different fractionation regimens (20 Gy in 5 fractions, 30 Gy in 10 fractions or 40 Gy in 20 fractions, determined by the facility at which they were treated), no difference was seen between regimens in terms of progression of disease, neurologic symptoms or toxicity (66).
A phase I dose-escalated accelerated hypofractionated trial was conducted by Caravatta and colleagues (68). Four fractions, ranging in fraction size from 3-4.5 Gy in 0.5 Gy increments, were delivered BID over 2 days. Only one patient experienced grade 3 acute neurologic toxicity with a median follow-up of 5 months. By 3 weeks after treatment, 76% of patients had resolution or improvement of their symptoms.
Treatment of patients with brain metastasis has changed since the publication of these trials. The majority of patients in the series described above had symptomatic brain metastasis. Today, many patients present simply as a result of a screening magnetic resonance imaging (MRI) and aggressive approaches for oligometastatic disease may be warranted depending on other disease and patient related factors. Since many patients, even if asymptomatic, present with multiple metastatic foci, the data is still very relevant. The majority of patients present with recursive partitioning analysis (RPA) class 2 and have a median survival of 4.5 months (69). For these patients 20 Gy in 5 fractions or supportive care alone are both reasonable options. There is currently no data to support less toxicity or better local control with longer courses of whole brain RT.
No data exists to support longer fractionated courses over shorter ones; however, there is value in adding surgery or stereotactic radiosurgery to whole brain radiation therapy in patients with good PS, single or oligometastatic (≤3) brain disease and controlled systemic disease. Post-operative radiosurgery or radiation boost can be considered in selected patients as can stereotactic radiosurgery alone. For patients whose expected survival is less than 3 months, either supportive care or whole brain radiation therapy are very reasonable approaches. The ASTRO brain metastasis guideline (70) reviews the indications and levels of evidence very well.
Discussion
Hypofractionated palliative radiation can dramatically improve QOL in patients with locally advanced or metastatic cancer. Due to the limited life span of such patients, and the potential difficulties that patients and their families have with transportation to treatment facilities, every effort should be made to decrease the burden of lengthy multi-fraction courses of radiation. Many centers currently provide same-day consultation, simulation and dose planning, and manage to deliver the first fraction of radiation as well. The rapid response RT programme at the Toronto-Sunnybrook Regional Cancer Center is one such example (71). In the setting of osseous metastasis, this may be the only visit necessary to achieve effective palliation. Additional fractions are typically required for treatment of non-osseous metastasis as detailed in this review. In many of the clinical scenarios detailed here, 2-fraction regimens provide equivalent relief of symptoms with minimal inconvenience to patients. Discussion of survival is complicated by the inclusion criteria of the studies and the practice patterns of different countries. In many of the studies, patients were included for palliative radiation treatment, due to limited resources and philosophy of care, who would be treated with curative intent in the US.
Effective palliation typically begins to occur within 7-10 days of initiation of radiation therapy, with full-effect within 4-6 weeks. Typically palliation is achieved for much of, if not all of the patient’s remaining lifespan. Given the timeframe to onset of relief, every effort should be made to avoid palliative radiation in those patients whose expected survival is less than 2 weeks. Physicians routinely overestimate life-expectancy which complicates the decision to begin palliative radiation. Palliative care professionals often cite lenghty multi-fraction radiation regimens as an impediment to referral for palliative radiation treatment. Radiation oncologists who consciously choose to offer shorter courses for appropriate end-of-life patients will certainly fit in with and better augment the efforts of their local hospice and palliative medicine teams.
Conclusions
Hypofractionated palliative radiation is well tolerated with minimal and self-limited side effects. The side effects that do occur are generally limited to the body region treated and last from a few days to a few weeks. Palliative RT is also generally cost-effective when compared to chemotherapy and bisphosphonates. Though the cost-effectiveness of palliative radiation therapy compares favorably to that of chemotherapy, it can still prove too costly for hospice programs. Many hospice programs limit or deny palliative radiation therapy, though it may benefit patients. Technologies designed to further reduce the incidence of rare but serious late effects have the potential to strain already tight budgets. Limiting the use of expensive techniques such as image-guidance and intensity modulated radiation therapy as well as limiting the number of fractions may help more patients gain access to needed palliation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Johnstone C, Lutz ST. External beam radiotherapy and bone metastases. Ann Palliat Med 2014;3:114-22.
- Coutard H. Principles of X-ray therapy of malignant diseases. Lancet 1934;2:1-8.
- Lutz ST, Chow EL, Hartsell WF, et al. A review of hypofractionated palliative radiotherapy. Cancer 2007;109:1462-70. [PubMed]
- Lutz S, Spence C, Chow E, et al. Survey on use of palliative radiotherapy in hospice care. J Clin Oncol 2004;22:3581-6. [PubMed]
- McCloskey SA, Tao ML, Rose CM, et al. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J 2007;13:130-7. [PubMed]
- Inoperable non-small-cell lung cancer (NSCLC): a Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer 1991;63:265-70. [PubMed]
- A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer 1992;65:934-41. [PubMed]
- Abratt RP, Shepherd LJ, Salton DG. Palliative radiation for stage 3 non-small cell lung cancer--a prospective study of two moderately high dose regimens. Lung Cancer 1995;13:137-43. [PubMed]
- Kramer GW, Wanders SL, Noordijk EM, et al. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol 2005;23:2962-70. [PubMed]
- Macbeth FR, Bolger JJ, Hopwood P, et al. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:167-75. [PubMed]
- Plataniotis GA, Kouvaris JR, Dardoufas C, et al. A short radiotherapy course for locally advanced non-small cell lung cancer (NSCLC): effective palliation and patients’ convenience. Lung Cancer 2002;35:203-7. [PubMed]
- Rees GJ, Devrell CE, Barley VL, et al. Palliative radiotherapy for lung cancer: two versus five fractions. Clin Oncol (R Coll Radiol) 1997;9:90-5. [PubMed]
- Senkus-Konefka E, Dziadziuszko R, Bednaruk-Młyński E, et al. A prospective, randomised study to compare two palliative radiotherapy schedules for non-small-cell lung cancer (NSCLC). Br J Cancer 2005;92:1038-45. [PubMed]
- Simpson JR, Francis ME, Perez-Tamayo R, et al. Palliative radiotherapy for inoperable carcinoma of the lung: final report of a RTOG multi-institutional trial. Int J Radiat Oncol Biol Phys 1985;11:751-8. [PubMed]
- Sundstrøm S, Bremnes R, Aasebø U, et al. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol 2004;22:801-10. [PubMed]
- Teo P, Tai TH, Choy D, et al. A randomized study on palliative radiation therapy for inoperable non small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 1988;14:867-71. [PubMed]
- Bezjak A, Dixon P, Brundage M, et al. Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NCIC CTG SC.15). Int J Radiat Oncol Biol Phys 2002;54:719-28. [PubMed]
- Bhatt ML, Mohani BK, Kumar L, et al. Palliative treatment of advanced non small cell lung cancer with weekly fraction radiotherapy. Indian J Cancer 2000;37:148-52. [PubMed]
- Cross CK, Berman S, Buswell L, et al. Prospective study of palliative hypofractionated radiotherapy (8.5 Gy x 2) for patients with symptomatic non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;58:1098-105. [PubMed]
- Donato V, Zurlo A, Bonfili P, et al. Hypofractionated radiation therapy for inoperable advanced stage non-small cell lung cancer. Tumori 1999;85:174-6. [PubMed]
- Lupattelli M, Maranzano E, Bellavita R, et al. Short-course palliative radiotherapy in non-small-cell lung cancer: results of a prospective study. Am J Clin Oncol 2000;23:89-93. [PubMed]
- Stevens MJ, Begbie SD. Hypofractionated irradiation for inoperable non-small cell lung cancer. Australas Radiol 1995;39:265-70. [PubMed]
- Vyas RK, Suryanarayana U, Dixit S, et al. Inoperable non-small cell lung cancer: palliative radiotherapy with two weekly fractions. Indian J Chest Dis Allied Sci 1998;40:171-4. [PubMed]
- Glare P, Sinclair C, Downing M, et al. Predicting survival in patients with advanced disease. Eur J Cancer 2008;44:1146-56. [PubMed]
- Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol 2008;26:4001-11. [PubMed]
- Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol 2011;1:60-71. [PubMed]
- Macbeth FR, Wheldon TE, Girling DJ, et al. Radiation myelopathy: estimates of risk in 1048 patients in three randomized trials of palliative radiotherapy for non-small cell lung cancer. The Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:176-81. [PubMed]
- Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys 2000;47:379-88. [PubMed]
- Srinivasan V, Brown CH, Turner AG. A comparison of two radiotherapy regimens for the treatment of symptoms from advanced bladder cancer. Clin Oncol (R Coll Radiol) 1994;6:11-3. [PubMed]
- Boulware RJ, Caderao JB, Delclos L, et al. Whole pelvis megavoltage irradiation with single doses of 1000 rad to palliate advanced gynecologic cancers. Int J Radiat Oncol Biol Phys 1979;5:333-8. [PubMed]
- Chafe W, Fowler WC, Currie JL, et al. Single-fraction palliative pelvic radiation therapy in gynecologic oncology: 1,000 rads. Am J Obstet Gynecol 1984;148:701-5. [PubMed]
- Halle JS, Rosenman JG, Varia MA, et al. 1000 cGy single dose palliation for advanced carcinoma of the cervix or endometrium. Int J Radiat Oncol Biol Phys 1986;12:1947-50. [PubMed]
- Hodson DI, Krepart GV. Once-monthly radiotherapy for the palliation of pelvic gynecological malignancy. Gynecol Oncol 1983;16:112-6. [PubMed]
- Jose CC, Price A, Norman A, et al. Hypofractionated radiotherapy for patients with carcinoma of the bladder. Clin Oncol (R Coll Radiol) 1999;11:330-3. [PubMed]
- Kynaston HG, Keen CW, Matthews PN. Radiotherapy for palliation of locally advanced prostatic carcinoma. Br J Urol 1990;66:515-7. [PubMed]
- McLaren DB, Morrey D, Mason MD. Hypofractionated radiotherapy for muscle invasive bladder cancer in the elderly. Radiother Oncol 1997;43:171-4. [PubMed]
- Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol 2001;82:167-71. [PubMed]
- Spanos W Jr, Guse C, Perez C, et al. Phase II study of multiple daily fractionations in the palliation of advanced pelvic malignancies: preliminary report of RTOG 8502. Int J Radiat Oncol Biol Phys 1989;17:659-61. [PubMed]
- Spanos WJ Jr, Perez CA, Marcus S, et al. Effect of rest interval on tumor and normal tissue response--a report of phase III study of accelerated split course palliative radiation for advanced pelvic malignancies (RTOG-8502). Int J Radiat Oncol Biol Phys 1993;25:399-403. [PubMed]
- Spanos WJ Jr, Wasserman T, Meoz R, et al. Palliation of advanced pelvic malignant disease with large fraction pelvic radiation and misonidazole: final report of RTOG phase I/II study. Int J Radiat Oncol Biol Phys 1987;13:1479-82. [PubMed]
- Wijkström H, Näslund I, Ekman P, et al. Short-term radiotherapy as palliative treatment in patients with transitional cell bladder cancer. Br J Urol 1991;67:74-8. [PubMed]
- Yan J, Milosevic M, Fyles A, et al. A hypofractionated radiotherapy regimen (0-7-21) for advanced gynaecological cancer patients. Clin Oncol (R Coll Radiol) 2011;23:476-81. [PubMed]
- Carrascosa LA, Yashar CM, Paris KJ, et al. Palliation of pelvic and head and neck cancer with paclitaxel and a novel radiotherapy regimen. J Palliat Med 2007;10:877-81. [PubMed]
- Caravatta L, Padula GD, Macchia G, et al. Short-course accelerated radiotherapy in palliative treatment of advanced pelvic malignancies: a phase I study. Int J Radiat Oncol Biol Phys 2012;83:e627-31. [PubMed]
- Lacarrière E, Smaali C, Benyoucef A, et al. The efficacy of hemostatic radiotherapy for bladder cancer-related hematuria in patients unfit for surgery. Int Braz J Urol 2013;39:808-16. [PubMed]
- Kim DH, Lee JH, Ki YK, et al. Short-course palliative radiotherapy for uterine cervical cancer. Radiat Oncol J 2013;31:216-21. [PubMed]
- Janjan NA, Breslin T, Lenzi R, et al. Avoidance of colostomy placement in advanced colorectal cancer with twice weekly hypofractionated radiation plus continuous infusion 5-fluorouracil. J Pain Symptom Manage 2000;20:266-72. [PubMed]
- Wong R, Thomas G, Cummings B, et al. The role of radiotherapy in the management of pelvic recurrence of rectal cancer. Can J Oncol 1996;6 Suppl 1:39-47. [PubMed]
- Scholten AN, Leer JW, Collins CD, et al. Hypofractionated radiotherapy for invasive bladder cancer. Radiother Oncol 1997;43:163-9. [PubMed]
- Chen AM, Vaughan A, Narayan S, et al. Palliative radiation therapy for head and neck cancer: toward an optimal fractionation scheme. Head Neck 2008;30:1586-91. [PubMed]
- Corry J, Peters LJ, Costa ID, et al. The ‘QUAD SHOT’--a phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol 2005;77:137-42. [PubMed]
- Paris KJ, Spanos WJ Jr, Lindberg RD, et al. Phase I-II study of multiple daily fractions for palliation of advanced head and neck malignancies. Int J Radiat Oncol Biol Phys 1993;25:657-60. [PubMed]
- Monnier L, Touboul E, Durdux C, et al. Hypofractionated palliative radiotherapy for advanced head and neck cancer: the IHF2SQ regimen. Head Neck 2013;35:1683-8. [PubMed]
- Das S, Thomas S, Pal SK, et al. Hypofractionated Palliative Radiotherapy in Locally Advanced Inoperable Head and Neck Cancer: CMC Vellore Experience. Indian J Palliat Care 2013;19:93-8. [PubMed]
- Kancherla KN, Oksuz DC, Prestwich RJ, et al. The role of split-course hypofractionated palliative radiotherapy in head and neck cancer. Clin Oncol (R Coll Radiol) 2011;23:141-8. [PubMed]
- Mohanti BK, Umapathy H, Bahadur S, et al. Short course palliative radiotherapy of 20 Gy in 5 fractions for advanced and incurable head and neck cancer: AIIMS study. Radiother Oncol 2004;71:275-80. [PubMed]
- Porceddu SV, Rosser B, Burmeister BH, et al. Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment—“Hypo Trial Radiother Oncol 2007;85:456-62. [PubMed]
- Weissberg JB, Pillsbury H, Sasaki CT, et al. High fractional dose irradiation of advanced head and neck cancer. Implications for combined radiotherapy and surgery. Arch Otolaryngol 1983;109:98-102. [PubMed]
- Borgelt BB, Gelber R, Brady LW, et al. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys 1981;7:587-91. [PubMed]
- Soliman H, Ringash J, Jiang H, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 2013;31:3980-6. [PubMed]
- Malik U, Mohiuddin M. External-beam radiotherapy in the management of liver metastases. Semin Oncol 2002;29:196-201. [PubMed]
- Scorsetti M, Alongi F, Clerici E, et al. Stereotactic body radiotherapy with flattening filter-free beams for prostate cancer: assessment of patient-reported quality of life. J Cancer Res Clin Oncol 2014;140:1795-800. [PubMed]
- Bydder S, Spry NA, Christie DR, et al. A prospective trial of short-fractionation radiotherapy for the palliation of liver metastases. Australas Radiol 2003;47:284-8. [PubMed]
- Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1-9. [PubMed]
- Borgelt B, Gelber R, Larson M, et al. Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1981;7:1633-8. [PubMed]
- Rades D, Lohynska R, Veninga T, et al. Evaluation of 2 whole-brain radiotherapy schedules and prognostic factors for brain metastases in breast cancer patients. Cancer 2007;110:2587-92. [PubMed]
- Gelber RD, Larson M, Borgelt BB, et al. Equivalence of radiation schedules for the palliative treatment of brain metastases in patients with favorable prognosis. Cancer 1981;48:1749-53. [PubMed]
- Caravatta L, Deodato F, Ferro M, et al. A phase I study of short-course accelerated whole brain radiation therapy for multiple brain metastases. Int J Radiat Oncol Biol Phys 2012;84:e463-8. [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [PubMed]
- Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012;2:210-25.
- Thavarajah N, Wong K, Zhang L, et al. Continued success in providing timely palliative radiation therapy at the Rapid Response Radiotherapy Program: a review of 2008-2012. Curr Oncol 2013;20:e206-11. [PubMed]


