Individual specialist physical activity assessment and intervention in advanced cancer patients on a palliative care ward; the 3STEPS-study
Background
Physical activity is beneficial not only in healthy people. There is accumulating evidence that it also causes positive effects in cancer patients (1), even in the palliative situation (2,3). Physiotherapy is a standard procedure on most palliative care wards. It aims to maintain mobility and quality of life in terminally ill patients and combines actives measures with passive interventions. Previous studies have demonstrated that physiotherapy is highly accepted in patients on palliative care wards and can be applied to high proportions of patients even within the last weeks and days of the patients’ life (4,5). Several studies reported that physical exercise and therapy have a beneficial effect on terminally ill cancer patients and impact their functional status (6-10). Montagnini and Cobbe investigated the utilization of physical exercise and therapy in a hospital- based palliative care unit and in hospice facility (7,8). The results showed that 37% and 65% of patients, respectively, were referred for a physical therapy evaluation. This may reflect the expanded role of physical exercise and therapy in terminally ill cancer patients. Physiotherapy is usually applied in a one-to-one model; structured assessment is performed in only 5–26% percent (9). Physical function is traditionally measured by physician rated scales for such as the Karnofsky performance status or according to the “The Eastern Cooperative Oncology Group” (ECOG) (11). The Tinetti-mobility test (TT) is the proposed assessment test for mobility by the German society for palliative care (12). In palliative care, self-report is considered as essential. Mobility is routinely self-reported in the weekly assessment using the palliative outcome scale (POS) or integrated POS (IPOS) respectively (13,14).
In advanced cancer patients, different standardized exercise interventions have been implemented and evaluated, despite the challenges caused by fluctuating trajectories and the impaired performance status of the patients. This has led to low inclusion and high drop-out rates in these trials and programs (15-17). Consequently, the majority of advanced cancer patients could not be reached with those interventions (18-20). This contrasts with the generally high willingness of patients to engage in physical exercise even at very advanced cancer stages (4,5).
In order to adapt the physiotherapeutic assessment and intervention to the specific capacities of advanced cancer patients receiving palliative care, who may be frail and impaired on different individual levels, a stepwise approach was chosen: Step 1: screening test, Step 2: specialist mobility assessment and Step 3: individualized activity intervention for those patients that are ready and capable to participate.
The activity intervention is derived from the “OTAGO Exercise Program’’, which was developed and validated in geriatric medicine, and has been proven to be feasible, safe and beneficial even in very old and frail patients (21,22).
This approach including a stepwise assessment and an intervention procedure stratified according to the patients’ individual physical capacities and might improve accessibility of patients on palliative care wards for physical activity programs.
Aim
The aim was to test the feasibility and effectiveness of an adapted activity assessment and intervention in patients on a palliative care ward and comparing the specialist physiotherapist assessment to standard assessments by other professions.
We present the following article in accordance with the STROBE Reporting Checklist (available at http://dx.doi.org/10.21037/apm-19-472).
Methods
The feasibility and outcome of an activity assessment and intervention was investigated.
Screening and assessment
All patients admitted to a specialized palliative care ward of a tertiary comprehensive cancer center between May 2017 and April 2018 underwent a screening procedure including routine assessment and specialist physiotherapeutic assessment.
Within 72 hours after admission to the palliative care ward, a basic assessment was performed by the specialist physiotherapist.
Imminently dying patients and patients whose psychological or physical state did not allow an assessment were excluded, as were patients that explicitly declined assessment.
The basic assessment encompasses the following five items:
- Patient is bedbound and in the dying phase;
- Patient is bedbound and mobilization is not possible;
- Patient declines mobilization;
- Patient is bedbound but mobilization is possible;
- Patient is mobile.
As standard procedure, physicians performed a routinely weekly performance status assessment (ECOG-Performance Status; 0–5 points). A nurse-led mobility assessment was done on a daily basis in order to measure nursing effort (ePA-AC = result-oriented nurse assessment for acute care; 3–12 points). Both assessments were documented in the electronic documentation system.
Patients themselves filled in the IPOS, which contains as an item a subjective patient reported mobility item (0–4 points, 0= best) on a weekly basis by paper and pen (14).
In patients in the group 4 and 5 of the basic assessment (“mobilization possible”), a TT was performed by the physiotherapist and documented in the electronic documentation system.
The TT encompasses 20 items and rates posture/position, movements like sitting, getting up standing and walking. Each item is rates with zero, one or two points. There are two subscales balance and gait. The maximal score of the test is 28 points (12).
Baseline measurements and intervention
At this point, informed consent for the interventional part (Step 3) was obtained and inclusion and exclusion criteria were checked. Pre-intervention measurements included physical function and global quality of life measured by the respective EORTC-QLQ-C30-scales, psychosocial distress was measured by the Distress Thermometer (DT) at begin of the intervention and at discharge (23).
The activity intervention was based on the “OTAGO Exercise Program’’ which combines strength and balance exercises. Patients were instructed to do three units per week; the length of the unit was 20–25 minutes (21,22).
Patients were encouraged to continue the program at home and a phone interview a month after discharge was done to check for adherence. Survival follow-up time was six months.
Data management and statistical analysis
Reasons for non-participation for Steps 2–3 were collected. Patient characteristics and disease specific information were obtained from the electronic medical record and pseudonymized. Data analysis was performed with SPSS, Version 22. Descriptive statistics was performed.
The study was approved by the local ethics committee in Hamburg (PV5515) Germany and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from the patients.
Results
Recruitment and demographics
In total 437 patients were admitted to the specialized palliative care ward during these 12 months. Mean length of stay on the ward was 9 days. Patients’ mean age was 67 years. Finally, 58% percent of patients died on the ward.
In 248 patients, a basic assessment was done, in 135 patients mobilization was possible; in 78 not-possible; 35 declined the assessment. The distribution of basic assessment scores was as follows: (I) n=36 (15%), (II) n=31 (13%), (III) n=43 (17%), (IV) n=58 (23%), and (V) n=80 (32%). Of those, 131 performed a TT and finally, 6 patients could be recruited for the interventional part of the study. The complete flow chart is displayed in (Figure 1).
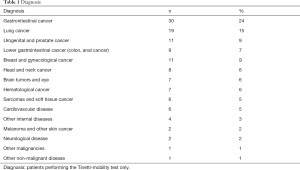
The 131 patients who underwent a TT had a median age of 63 years (range, 22–95 years); Gender balance was 58 female vs. 73 male. Median length of stay on the ward was 13 days (SD 7 days). During the hospitalization, 34 of the 129 patients died on the ward (26%) and 48 patients (54%) were discharged at home. The underlying disease groups are displayed in Table 1.
Full table
TT
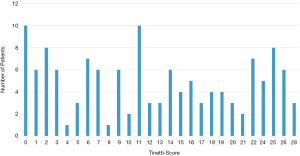
The median score in the 131 patients in the TT was 12 (SD 9) of 28. The distribution of the frequencies of the scores is displayed in (Figure 2).
Comparison of four assessment methods
The 131 patients, who underwent a TT, had the following mean scores in the other assessment methods; basic-assessment physiotherapy: 4.57 (SD 0.5); ECOG-Performance Status; (0–5 points): ECOG 2.9 (SD 0.6); ePA-AC (3–12 points): 8.2 (SD 2.3) and IPOS (0–4 points, 0= best): 2.84 (SD 1.0). The correlations between assessment methods are displayed in Table 2.

Full table
Intervention
Six patients gave informed consent to the intervention and four of them completed the intervention. Two of them reported continuing the program at home.
The characteristics of these six patients are displayed in Table 3.

Full table
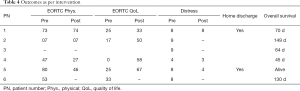
In the patients who were able to complete the intervention, a reduction of distress and increase in quality of life was seen (Table 4).
Full table
Discussion
In a stepwise procedure, a systematic assessment concerning mobility was started in all patients admitted to a specialized palliative care ward. However, in only half of the patients, the specific specialist basic assessment was feasible and the TT could be performed in only about a quarter of the patients. In those patients, the median score in the TT was relatively low. Finally, only six patients gave consent to intervention part. At least, in the four patients who completed the intervention an improvement in outcomes was seen. The most common reason for non-participation were “cognition” (31%) and “physical” (27%). Due to the low inclusion rate, the study was closed after one year.
When comparing the four assessment methods, only low to moderate correlations could be detected. The highest correlation was between physiotherapist and nurses, which might be the two most objectively measured assessment, the correlation between ECOG test and IPOS was the lowest and not significant, which might be due to the opposition between the most subjective assessments. The distribution in the TT was widely spread over the scale, which indicates the difficulty to make recommendations for this heterogeneous group concerning physical activity.
Despite the precautions of an individualized stepwise approach for the intervention, our results lie in line with previous studies, which reported low accrual and high attrition rates: a group in Germany reported similar challenges in feasibility in a comparable setting (18). In a case series for a home-based functional walking program, of 524 patients screened, only 9 were included and only 3 completed the program (24). In the hospice setting, only half of the patients completed a nine-session exercise program despite excellent feedback concerning the program (25). In a large randomized controlled trial of an eight-week exercise intervention in palliative care, survival of completers was 16.2 months, which represents a selection of patients much earlier in the disease trajectory than in our population (16).
The main limitation was that both assessment and intervention were too burdensome in this population of patients admitted to a palliative care ward, even though the instrument and programs are either officially proposed for palliative care or designed for a geriatric and therefore frail population. One barrier for trial participation might be study procedures including informed consent, regular assessments and questionnaires rather than physical activation on demand. Due to the small numbers, the results represent more a case series than an intervention study. A limitation concerning the assessment part is that the mobility assessment done by the nursing staff is not validated for this purpose, it is intended to quantify nursing effort.
Generally, physiotherapy in palliative care seems useful to complement activation efforts with measures for symptom control such respiratory therapy or manual lymphatic drainage or more passive elements, such as massage or relaxation. If activation is the goal in these frail patient population, it might be more suitable in the setting of repeated offers rather than within a fixed program that must be completed.
Patients at an earlier stage of disease might be a more suitable population for physical activity interventions. At this late stage physical activity interventions might be questionable.
Conclusions
Despite systematic stepwise-adapted screening, only six patients could be accrued to the individually adapted activity intervention. Mainly, physical performance and cognition of the patients admitted to the specialized palliative care ward was even lower than expected. However, in these selected patients able to adhere to the program, a positive effect was seen. A multiprofessional assessment approach for low activity and bed-bound patients might be more suitable in this population.
Acknowledgments
Funding: This study was supported by a grant of the Eppendorfer Krebs- & Leukämiehilfe e.V. Hamburg Germany
Footnote
Reporting Checklist: The authors have completed the STROBE Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-19-472
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-472). Dr. CB reports personal fees from Merck KGaA, Sanofi, Roche, Bristol-Myers Squibb, AstraZeneca, Merck Sharp Dohme, Lilly/ImClone, Merck Serona, Sanofi, Bayer Schering Pharma, Merk Sharp & Dohme, GSO, ADK Health Insurance, Merck Serono, Sanofi, Pfizer, Bristol-Myers Squibb, grants from Abbvie, ADC Therapeutics, Agile Therapeutics, Alexion Pharmaceuticals, Amgen, Apellis Pharmaceuticals, Astellas Pharma, AstraZeneca, Bayer, BerGenBio, Blueprint Medicines, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eisai, Gilead Sciences, Glycotope GmbH, GlaxoSmithKline, Incyte, IO Biotech, Isofol Medical, Janssen-Cilag, Karyopharm Therapeutics, Lilly, Millennium, MSD, Nektar, Novartis, Rafael Pharmaceuticals, Roche, Springworks Therapeutics, Taiho Pharmaceuticals outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the local ethics committee in Hamburg (PV5515) Germany and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travier N, Velthuis MJ, Steins Bisschop CN, et al. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med 2015;13:121. [Crossref] [PubMed]
- Lowe SS, Watanabe SM, Baracos VE, et al. Associations Between Physical Activity and Quality of Life in Cancer Patients Receiving Palliative Care: A Pilot Survey. J Pain Symptom Manage 2009;38:785-96. [Crossref] [PubMed]
- Litterini AJ, Fieler VK, Cavanaugh JT, et al. Differential Effects of Cardiovascular and Resistance Exercise on Functional Mobility in Individuals With Advanced Cancer: A Randomized Trial. Arch Phys Med Rehabil 2013;94:2329-35. [Crossref] [PubMed]
- Oechsle K, Jensen W, Schmidt T, et al. Physical activity, quality of life, and the interest in physical exercise programs in patients undergoing palliative chemotherapy. Support Care Cancer 2011;19:613-9. [Crossref] [PubMed]
- Lowe SS, Watanabe SM, Baracos VE, et al. Physical activity interests and preferences in palliative cancer patients. Support Care Cancer 2010;18:1469-75. [Crossref] [PubMed]
- López-Sendín N, Alburquerque-Sendín F, Cleland JA, et al. Effects of Physical Therapy on Pain and Mood in Patients with Terminal Cancer: A Pilot Randomized Clinical Trial. J Altern Complement Med 2012;18:480-6. [Crossref] [PubMed]
- Montagnini M, Lodhi M, Born W. The Utilization of Physical Therapy in a Palliative Care Unit. J Palliat Med 2003;6:11-7. [Crossref] [PubMed]
- Cobbe S, Kennedy N. Physical Function in Hospice Patients and Physiotherapy Interventions: A Profile of Hospice Physiotherapy. J Palliat Med 2012;15:760-7. [Crossref] [PubMed]
- Ebel S, Langer K. The role of the physical therapist in hospice care. Am J Hosp Palliat Care 1993;10:32-5. [Crossref] [PubMed]
- Porock D, Kristjanson LJ, Tinnelly K, et al. An Exercise Intervention for Advanced Cancer Patients Experiencing Fatigue: A Pilot Study. J Palliat Care 2000;16:30-6. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Tinetti ME. Performance-Oriented Assessment of Mobility Problems in Elderly Patients. J Am Geriatr Soc 1986;34:119-26. [Crossref] [PubMed]
- Bausewein C, Fegg M, Radbruch L, et al. Validation and Clinical Application of the German Version of the Palliative Care Outcome Scale. J Pain Symptom Manage 2005;30:51-62. [Crossref] [PubMed]
- Schildmann EK, Groeneveld EI, Denzel J, et al. Discovering the hidden benefits of cognitive interviewing in two languages: The first phase of a validation study of the Integrated Palliative care Outcome Scale. Palliat Med 2016;30:599-610. [Crossref] [PubMed]
- Grande AJ, Silva V, Maddocks M. Exercise for cancer cachexia in adults: Executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle 2015;6:208-11. [Crossref] [PubMed]
- Oldervoll LM, Loge JH, Lydersen S, et al. Physical Exercise for Cancer Patients with Advanced Disease: A Randomized Controlled Trial. Oncologist 2011;16:1649-57. [Crossref] [PubMed]
- Cheville AL, Kollasch J, Vandenberg J, et al. A Home-Based Exercise Program to Improve Function, Fatigue, and Sleep Quality in Patients With Stage IV Lung and Colorectal Cancer: A Randomized Controlled Trial. J Pain Symptom Manage 2013;45:811-21. [Crossref] [PubMed]
- Siemens W, Wehrle A, Gaertner J, et al. Implementing a home-based exercise program for patients with advanced, incurable diseases after discharge and their caregivers: lessons we have learned. BMC Res Notes 2015;8:509. [Crossref] [PubMed]
- Chinn DJ, White M, Howel D, et al. Factors associated with non-participation in a physical activity promotion trial. Public Health 2006;120:309-19. [Crossref] [PubMed]
- Santa Mina D, Petrella A, Currie KL, et al. Enablers and barriers in delivery of a cancer exercise program: the Canadian experience. Curr Oncol 2015;22:374-84. [Crossref] [PubMed]
- Campbell AJ, Robertson MC, Gardner MM, et al. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 1997;315:1065-9. [Crossref] [PubMed]
- Robertson MC. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 2: Controlled trial in multiple centres. BMJ 2001;322:701-4. [Crossref] [PubMed]
- Mehnert A, Müller D, Lehmann C, et al. Die deutsche Version des NCCN Distress-Thermometers. Zeitschrift für Psychiatrie, Psychologie und Psychotherapie 2006;54:213-23. [Crossref]
- Lowe SS, Watanabe SM, Baracos VE, et al. Home-based functional walking program for advanced cancer patients receiving palliative care: a case series. BMC Palliat Care 2013;12:22. [Crossref] [PubMed]
- Talbot Rice H, Malcolm L, Norman K, et al. An evaluation of the St Christopher's Hospice rehabilitation gym circuits classes: Patient uptake, outcomes, and feedback. Prog Palliat Care 2014;22:319-25. [Crossref] [PubMed]