Predictors for the clinical benefit of anti-PD-1/PD-L1 therapy in advanced gastroesophageal cancer: a meta-analysis of clinical trials
Introduction
Gastroesophageal cancer is the top ten leading cause of cancer-related death worldwide (1,2). Surgery significantly improved the survival of the patients with early-stage disease. However, the treatments for patients with locally advanced or metastatic disease are far away from satisfying (3). Recently, the success of several immune check inhibitors (ICIs) brought the treatments of cancer into the immunotherapy era (4). Immune checkpoint inhibitors (ICIs) target the key regulators who can help the tumor cells escape from the immune attack so that it could enhance the cytotoxic activity of immune cells against the tumor cells (5,6). Among the regulators, the program-death 1 (PD-1) and program death legend 1 (PD-L1) are being widely investigated (7). The FDA has approved two kinds of anti-PD-1 antibodies (nivolumab and pembrolizumab) and three types of anti-PD-L1 antibodies (avelumab, atezolizumab, and durvalumab) for the treatment of several cancers (8).
Several phase three clinical trials have proved the safety and efficacy of anti-PD-1/anti-PD-L1 therapy in advanced gastroesophageal cancer (9-11). However, a meta-analysis of published clinical trials showed that the overall objective response rate (ORR) in gastroesophageal cancer patients who received anti-PD-1/anti-PD-L1 therapy was only 10% (8). This ratio reveals that it is imperative to identify reliable predictors to help the clinicians screen out the patients who are more likely to benefit from the therapy.
The previously published meta-analyses have indicated that gender, PD-L1 expression level, and high microsatellite instability are associated with the efficacy of the ICIs (12,13). However, these conclusions are based on the comparison between the patients who received ICIs, and the patients received chemotherapy. The accurate predictors should not come from such comparisons. What is more, anti-PD-1/PD-L1 therapy and anti-CTLA-4 therapy were pooled together in these meta-analyses. It is not scientific and reliable. So we conducted this meta-analysis in which only the patients received anti-PD-1/PD-L1 therapy would be included, and all comparisons were made among these patients. Therefore, we can identify the factors with which the gastroesophageal cancer patients would be more likely to benefit from the anti-PD-1 or anti-PD-L1 therapy scientifically. We present the following article in accordance with the PRISMA Reporting Checklist (available at http://dx.doi.org/10.21037/apm-19-430a).
Methods
The study was carried out according to the Cochrane handbook for systemic reviews of intervention, and the results were reported following the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) guideline (14). The study protocol was registered with PROSPERO.
Systematic research of the potentially relevant publications was performed in the online database of Pubmed, Medline, EMBASE, and Cochrane Central Register of controlled trials on 21st July 2019. The searching strategy was consisted of following terms: (immune checkpoint inhibitor OR ICI OR immunotherapy OR PD-1 OR PD-L1 OR Nivolumab OR Pembrolizumab OR Avelumab OR Atezolizumab OR Durvalumab OR Tremelimumab OR Relatlimab) AND (esophageal OR esophagus OR oesophageal OR oesophagus OR gastric OR stomach OR gastroesophageal OR gastro-oesophageal OR GEJ OR esophagogastric OR EGJ) AND (cancer OR carcinoma OR neoplasm OR tumor OR tumour).
Inclusion and exclusion criteria
Inclusion criteria: (I) patients received anti-PD-1 or anti-PD-L1 therapy in the study. (II) The study focused on patients with esophageal, gastroesophageal, or gastric cancer. (III) The study compared the short-term or long-term outcomes of the immunotherapy according to the patients’ characteristics.
Exclusion criteria: (I) The study design is not a clinical trial. (II) Following publication types: review, meta-analysis, case report, study protocol, conference abstract, letter, and reply. (III) When duplicate data occurred, the study enrolled more patients would be included.
Study screening and data extraction
The primary screening was done by reading the titles and abstracts of the studies. Most of the irrelevant studies were excluded in this step. Then, the second round screening was performed by reading the full texts of the left, potentially relevant studies. After that, we started to extract relevant data to finally confirm the studies which could be included in this meta-analysis. The following baseline characteristics data of the studies were collected: name of the first author, publication year, trial code and phase, treatment strategy, the number of participants. The major outcomes including the objective response rate (ORR), disease control rate (DSR), overall survival (OS) and progression-free survival (PFS) were collected according to the patients’ characteristics such as gender, age, and PD-L1 expression level.
All the work above was accomplished by two authors (Zhuo and Deng) independently and then checked with each other. Disagreements were resolved by discussing it with another author (Lin).
Quality assessment
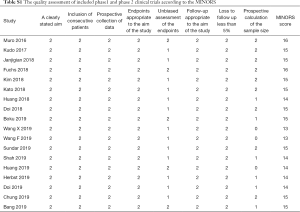
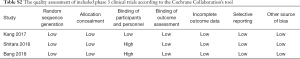
The Cochrane Collaboration’s tool published in the Cochrane Handbook (version 5.3) which contained seven items was used to evaluate the quality of phase three randomized clinical trials while the Methodological Index for Non-randomized Studies (MINORS) (15) was used to assess the quality of phase 1 or phase 2 clinical trials.
Statistical analysis
The Review Manager Version 5.3 and STATA Version 12.0 software (Stata Corporation, College Station, TX, USA) were used to perform the data analysis. Odds ratio (OR) was used in the comparison of dichotomous data. We used I2 as an indicator of heterogeneity. I2 <25%, 25% ≤ I2 <50% and I2 ≥50% indicated low, moderate and high heterogeneity. When high heterogeneity was detected, a random-effects model was adopted; otherwise, a fixed-effects model was adopted. Begg’s and Egger’s tests were used to detect publication bias. A P value of less than 0.05 was considered to be statistically significant.
Results
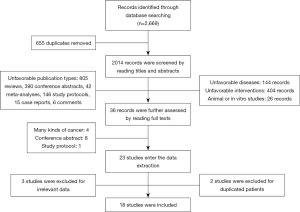
A total of 2,669 records were identified through the online database searching. The procedures of study screening were showed in Figure 1. After the removal of 655 duplicated records, two thousand and fourteen records entered the first round screening. Then, by reading the titles and abstracts, 1,404 records were excluded for unfavorable publication types (reviews, meta-analyses, conference abstracts, study protocols, case reports, and comments). One hundred and forty-four records focused on other diseases were also excluded. Another 404 records did not prescribe the anti-PD-1/anti-PD-L1 therapy to the participants who were excluded as well. Twenty-six animal or in vitro studies were also removed. After that, thirty-six records entered the third round screening. By reading the full texts, one protocol, four studies enrolled several kinds of cancers, and eight conference abstracts were further excluded.
Twenty-three studies entered data extraction. Two studies were excluded for duplicate data, and three studies with no related outcomes for this meta-analysis were also removed. Finally, eighteen studies from seventeen clinical trials were included in the final analysis.
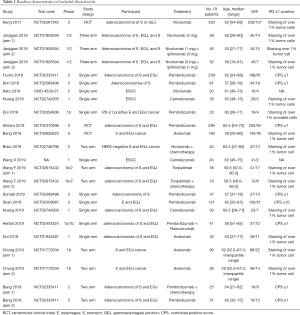
Table 1 showed the baseline characteristics of the included studies. Three of them were phase three randomized clinical trials (9-11) while the others were phase 1 or phase 2 clinical trials (16-30). The quality assessment of the enrolled studies was available in the appendix (Table S1 and Table S2). One thousand and nine hundred ninety-eight patients with advanced gastroesophageal cancer receiving anti-PD-1 or anti-PD-L-1 therapy were enrolled totally. Five anti-PD-1 or anti-PD-L-1 antibodies (pembrolizumab, nivolumab, camrelizumab, avelumab, toripalimab) were used in these trials.
Full table
Full table
Full table
Predictors of anti-PD-1/anti-PD-L1 therapy response
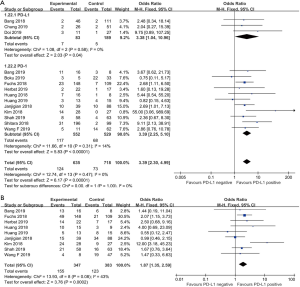
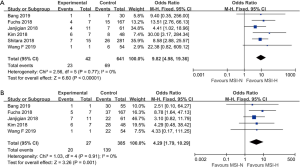
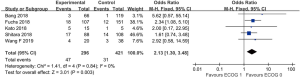
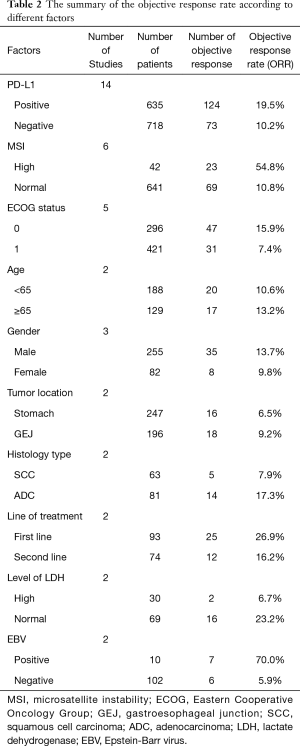
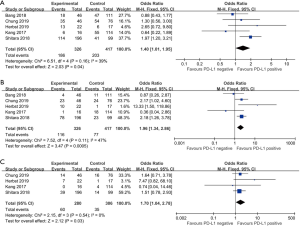
The patients who achieved a complete response (CR) or partial response (PR) rate were defined as having an objective response to the therapy, and those achieved CR, PR, and stable disease (SD) were defined as having a disease control. Fourteen studies compared the objective response rate (ORR) of the anti-PD-1/anti-PD-L1 therapy between PD-L1 positive and PD-L1 negative patients. The overall ORR in PD-L1 positive patients was 19.5%, while it was 10.2% in PD-L1 negative patients. Furthermore, the difference reached statistically significant in the pooled analysis (OR =3.39, 95% CI: 2.30, 4.99, P<0.001, Figure 2A). The results remain the same in the subgroup analysis of anti-PD-1 therapy and anti-PD-L1 therapy (Figure 2A). Nine studies reported the disease control rate (DCR) between the PD-L1 positive and negative patients, and all the nine studies were about anti-PD-1 therapy. The analysis also showed the PD-L1 positive patients were more likely to have a disease control after the treatment (OR =1.87, 95% CI: 1.35, 2.59, P<0.001, Figure 2B). The DCR was 44.7% and 32.1% in PD-L1 positive and negative patients, respectively. Six studies conducted a comparison between patients with MSI-H (microsatellite instability high) and those with MSI-N (microsatellite instability normal). And all the patients enrolled were with adenocarcinoma. The overall ORR in patients with MSI-H was 54.8% while it was 10.8% in MSI-N patients (OR =9.82, 95% CI: 4.98, 19.36, P<0.001, Figure 3A). The DCR was also significantly higher in the MSI-H patients (74.0% versus 36.1%, P=0.001, Figure 3B). Five studies conducted the comparison of the anti-PD-1/anti-PD-L1 therapy response between patients with an ECOG performance status of zero and one. The pooled analysis indicated a statistically significant higher ORR in patients with an ECOG performance status of zero (OR =2.13, 95% CI: 1.30, 3.48, P=0.003, Figure 4). Only two studies reported the DCR in patients with ECOG performance status of zero and one. The DCR was 48.6% and 41.9% in patients with ECOG performance status of zero and one, respectively. Three studies compared the ORR of the therapy according to gender. Nonetheless, the male patients had a higher ORR (13.7% versus 9.8%), the difference did not reach statistically significant (OR =1.45, 95% CI: 0.64, 3.30, P=0.37).
The results of other factors whose data were available only in two studies and unsuitable for pooled analysis because of limited sample size and high heterogeneity were summary in Table 2.
Full table
Predictors of long-term survival
Four studies were enrolled in the comparison of the 6-month progression-free survival (PFS) rate of the anti-PD-1/anti-PD-L1 therapy between PD-L1 positive and negative patients. The 6-month PFS rate was 18.4% in PD-L1 positive patients, while it was 9.0% in PD-L1 negative patients. Moreover, the difference reached statistically significant (OR =2.07, 95% CI: 1.23, 3.48, P=0.006, Figure 5A). So did the 12- and 18-month PFS. The 12-month PFS rate was 11.0% and 3.3% in PD-L1 positive and negative patients respectively (OR =2.57, 95% CI: 1.23, 5.36, P=0.01, Figure 5B). The 18-month PFS was 7.7% and 1.0% in PD-L1 positive and negative patients respectively (OR =4.55, 95% CI: 1.42, 14.63, P=0.01, Figure 5C).
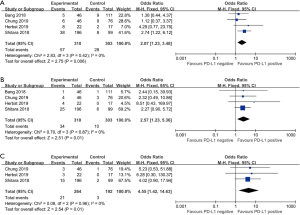
Five studies compared the overall survival (OS) between the PD-L1 positive and negative patients. The 6-month OS rate in PD-L1 positive patients was 57.1% while it was 48.7% in the PD-L1 negative patients (OR =1.40, 95% CI: 1.01, 1.95, Figure 6A). The 12-month OS rate was also statistically significant higher in the PD-L1 positive patients (35.6% versus 18.5%, OR =1.96, 95% CI: 1.34, 2.86, P<0.01, Figure 6B). The PD-L1 positive patients had a statistically significant higher 18-month PFS rate than the PD-L1 negative patients as well. The 18-month OS rate was 21.4% and 11.4% in PD-L1 positive and negative patients respectively (OR =1.70, 95% CI: 1.04, 2.78, P=0.03, Figure 6C).
Heterogeneity and publication bias
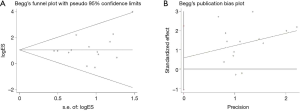
Among all the pooled analyses, only the disease control rate (DCR), the 6-month OS rate, and the 12-month OS rate according to the PD-L1 expression showed moderate heterogeneity while the left analyses were with low heterogeneity. The Begg’s (P=0.324) and Egger’s test (P=0.461) detected no publication bias in the comparison of ORR according to the PD-L1 expression level (Figure 7).
Discussion
Our study was the first meta-analysis to explore the predictors of the response and long-term survival of anti-PD-1/anti-PD-L1 therapy in gastroesophageal cancer patients. It revealed that patients with the high expression of PD-L1, high microsatellite instability, and ECOG performance status of zero were more likely to achieve an objective response from the anti-PD-1/anti-PD-L1 therapy. Furthermore, the therapy had a better performance in improving the OS and PFS in PD-L1 positive patients than the PD-L1 negative patients.
The PD-1 antibodies target at the PD-1 on the immune cells while the PD-L1 antibodies target ar the PD-L1 on the tumor cells (31,32). So it was not surprising that patients with the PD-L1 positive tumors had a higher ORR and DCR than the PD-L1 negative patients when receiving the anti-PD-1/anti-PD-L1 therapy. The ORR in PD-L1 positive patients was almost twice as high as PD-L1 negative patients in the pooled analysis. A phase three clinical trial showed the ORR in patients with PD-L1 CPS of one, or higher was 16% while it was 24.5% in patients with PD-L1 CPS of ten or higher in gastroesophageal cancer (10). Huang et al. also reported the ORR could reach as high as 46.5% in esophageal squamous cell carcinoma patients with over 5% PD-L1 staining tumor cell (21). These results indicate that the efficacy of anti-PD-1/anti-PD-L1 therapy has a positive relationship with the expression level of the PD-L1. A meta-analysis showed that the high expression of PD-L1 was associated with poorer overall survival in ESCC (33). However, our study showed it was exactly these patients who could achieve a better OS and PFS from the anti-PD-1/anti-PD-LA therapy. It proved the efficacy of the therapy and indicated the predictive value of the expression level of PD-L1 in the therapy indirectly.
The reported overall proportion of patients with MSI-H ranged from 5% to 9% in gastroesophageal cancer (34-36). In our study, 42 out of 683 patients (6.1%) were in MSI-H status, and twenty-three achieved objective responses (54.8%). The ORR of MSI-H patients was much higher than the MSS patients. The MSI-H patients also had a significantly higher DCR (74.1%). The higher efficacy of ICIs in MSI-H patients was also observed in other cancers (37,38). This may associate with the upregulation of immune checkpoint proteins in the MSI-H patients, including PD-1 and PD-L1 (37,39). Although only a small part of the patients are in MSI-H status, once it occurred, the patients have a great chance to benefit from the anti-PD-1/anti-PD-LA therapy. In consideration of this, the FDA has approved pembrolizumab for the treatments of metastatic MSI-H tumors, irrespective of the site of origin (40). The MSI-H status is the most predictive single factor of the response to anti-PD-1/anti-PD-LA therapy in gastroesophageal cancer now.
The ECOG performance status is an assessment of the patients’ functional status (41). A better ECOG performance status is associated with better clinical outcomes in cancer patients, irrespective of the type of systemic therapy (42,43). All the five studies enrolled in the analysis of ORR of anti-PD-1/anti-PD-L1 therapy according to the ECOG performance status showed a higher ORR in patients with a score of zero than those with a score of one. Moreover, the pooled analysis showed a statistically significant difference. Wang et al. showed that gastroesophageal cancer patients with a better ECOG performance status could also get a better overall survival from the therapy (29).
Particular attention should be paid to several factors such as EBV infection, line of treatments, level of LDH, and histology type, which is seldom reported in the published trials. Although we are unable to prove the predictive value of them statistically, the high ORR in patients with these characteristics should not be ignored by future studies.
There are some limitations to our meta-analysis. Firstly, only three of the included studies were the phase three clinical trial, while the others were phase one or two clinical trials. This brought down the evidence level of our results in some way. Secondly, the analyses of the long-term survival according to the microsatellite status and ECOG performance status were unable to perform with the available data. So if the patients with MSI-H and better ECOG performance status would have a better long-term survival from the anti-PD-1/anti-PD-L1 therapy are needed to be further proved.
In summary, this meta-analysis showed the PD-L1 expression level, microsatellite status, and ECOG performance status could be the predictors of the efficacy of anti-PD-1/anti-PD-L1 therapy in advanced gastroesophageal cancer. However, the predictive value of the single factor is limited. We are looking forward to the constriction of the predicting models based on these predictors in future studies.
Acknowledgments
We would like to thank Mr. Shi-De Wu from the High School Attached to Northeast Normal University for his linguistic assistance to this manuscript.
Funding: The National Natural Science Foundation of China supported the study (Grant number: 81672291, 31071210).
Footnote
Reporting Checklist: The authors have completed the PRISMA Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-19-430a
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-430a). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This is a meta-analysis. It does not involve any ethical or informed consent problems. This article does not contain any studies with human participants performed by any of the authors.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. [Crossref] [PubMed]
- Kelly RJ. The emerging role of immunotherapy for esophageal cancer. Curr Opin Gastroenterol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med 2016;14:73. [Crossref] [PubMed]
- Tian Y, Zhai X, Han A, et al. Potential immune escape mechanisms underlying the distinct clinical outcome of immune checkpoint blockades in small cell lung cancer. J Hematol Oncol 2019;12:67. [Crossref] [PubMed]
- Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018;48:434-52. [Crossref] [PubMed]
- Ku GY. The Current Status of Immunotherapies in Esophagogastric Cancer. Hematol Oncol Clin North Am 2019;33:323-38. [Crossref] [PubMed]
- Ni X, Xing Y, Sun X, et al. The safety and efficacy of anti-PD-1/anti-PD-L1 antibody therapy in the treatment of previously treated, advanced gastric or gastro-oesophageal junction cancer: A meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol 2020;44:211-22. [Crossref] [PubMed]
- Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018;29:2052-60. [Crossref] [PubMed]
- Shitara K, Ozguroglu M, Bang Y-J, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123-33. [Crossref] [PubMed]
- Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- El-Osta H, Jafri S. Predictors for clinical benefit of immune checkpoint inhibitors in advanced non-small-cell lung cancer: a meta-analysis. Immunotherapy 2019;11:189-99. [Crossref] [PubMed]
- Chen C, Zhang F, Zhou N, et al. Efficacy and safety of immune checkpoint inhibitors in advanced gastric or gastroesophageal junction cancer: a systematic review and meta-analysis. OncoImmunology 2019;8:e1581547. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [PubMed]
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712-6. [Crossref] [PubMed]
- Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 2019;30:250-8. [Crossref] [PubMed]
- Chung HC, Arkenau HT, Lee J, et al. Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer 2019;7:30. [Crossref] [PubMed]
- Doi T, Iwasa S, Muro K, et al. Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN Solid Tumor JPN trial. Gastric Cancer 2019;22:817-27. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- Herbst RS, Arkenau HT, Santana-Davila R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol 2019;20:1109-23. [Crossref] [PubMed]
- Huang J, Xu B, Mo H, et al. Safety, Activity, and Biomarkers of SHR-1210, an Anti-PD-1 Antibody, for Patients with Advanced Esophageal Carcinoma. Clin Cancer Res 2018;24:1296-304. [Crossref] [PubMed]
- Huang J, Mo H, Zhang W, et al. Promising efficacy of SHR-1210, a novel anti-programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer 2019;125:742-9. [Crossref] [PubMed]
- Janjigian Yy BJCEKJWAPASPOPAPKJDEJDB. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol 2018;36:2836. [Crossref] [PubMed]
- Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449-58. [Crossref] [PubMed]
- Shah MA, Kojima T, Hochhauser D, et al. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients with Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol 2019;5:546-50. [Crossref] [PubMed]
- Wang F, Wei XL, Wang FH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol 2019;30:1479-86. [Crossref] [PubMed]
- Kato R, Yamasaki M, Urakawa S, et al. Increased Tim-3(+) T cells in PBMCs during nivolumab therapy correlate with responses and prognosis of advanced esophageal squamous cell carcinoma patients. Cancer Immunol Immunother 2018;67:1673-83. [Crossref] [PubMed]
- Bang YJ, Kang YK, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 2019;22:828-37. [Crossref] [PubMed]
- Wang X, Zhang B, Chen X, et al. Lactate dehydrogenase and baseline markers associated with clinical outcomes of advanced esophageal squamous cell carcinoma patients treated with camrelizumab (SHR-1210), a novel anti-PD-1 antibody. Thorac Cancer 2019;10:1395-401. [PubMed]
- Doi T, Piha-Paul SA, Jalal SI, et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol 2018;36:61-7. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Sebastian M, Schroder A, Scheel B, et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol Immunother 2019;68:799-812. [Crossref] [PubMed]
- Guo W, Wang P, Li N, et al. Prognostic value of PD-L1 in esophageal squamous cell carcinoma: a meta-analysis. Oncotarget 2017;9:13920-33. [Crossref] [PubMed]
- Haag GM, Czink E, Ahadova A, et al. Prognostic significance of microsatellite-instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy. Int J Cancer 2019;144:1697-703. [Crossref] [PubMed]
- Polom K, Marano L, Marrelli D, et al. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg 2018;105:159-67. [Crossref] [PubMed]
- Campanella NC, Lacerda CF, Berardinelli GN, et al. Presence of microsatellite instability in esophageal squamous cell carcinoma associated with chagasic megaesophagus. Biomark Med 2018;12:573-82. [Crossref] [PubMed]
- Dudley JC, Lin MT, Le DT, et al. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res 2016;22:813-20. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Chang L, Chang M, Chang HM, et al. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Appl Immunohistochem Mol Morphol 2018;26:e15-e21. [PubMed]
- De Mello RA, Lordick F, Muro K, et al. Current and Future Aspects of Immunotherapy for Esophageal and Gastric Malignancies. Am Soc Clin Oncol Educ Book 2019;39:237-47. [Crossref] [PubMed]
- Young J, Badgery-Parker T, Dobbins T, et al. Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage 2015;49:258-64. [Crossref] [PubMed]
- Huemer F, Lang D, Westphal T, et al. Baseline Absolute Lymphocyte Count and ECOG Performance Score Are Associated with Survival in Advanced Non-Small Cell Lung Cancer Undergoing PD-1/PD-L1 Blockade. J Clin Med 2019;8:1014. [Crossref] [PubMed]
- Haus R, Janssen S, Schild SE, et al. Eastern Cooperative Oncology Group Performance Score Is Associated With Survival After Radiotherapy of Bone Metastases from Prostate Cancer. In Vivo 2020;34:679-82. [Crossref] [PubMed]