
Prediction of the significance in the improvement of depression symptoms of amisulpride in the treatment of schizophrenia: an 8-week case-control study
Introduction
At present, antipsychotic drug treatment is an effective method widely used in the clinical symptom control of schizophrenia, which significantly improves the remission rate of psychiatric symptoms and the discharge rate of patients (1). Drug treatment should be systematic and standardized, emphasizing early, adequate, and a full course of treatment, and paying attention to the principle of single drug use and individualized drug administration (2). Aminosulpiride is a benzamide used to treat acute or chronic schizophrenia with positive symptoms (such as delirium, hallucination, and cognitive impairment) and/or negative symptoms (such as delayed response, emotional apathy, and social withdrawal), as well as schizophrenia characterized by negative symptoms (3). Many studies have examined the efficacy and safety of amisulpride in the treatment of schizophrenia. However, for chronic schizophrenia, managing patients’ mental symptoms through drug administration is a long process. During treatment, the drug efficacy should also be evaluated and actively predicted, so that the medication could be adjusted in timely fashion to ensure effective treatment (4). Some researchers believe that early improvement of depression symptoms in patients has a certain predictive effect on the recovery of symptoms after drug treatment for schizophrenia (5). To further validate this view and to explore the predictors of amisulpride in the treatment of schizophrenia, the following study was conducted.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1702).
Methods
General information
The study subjects included383 patients with schizophrenia who were admitted to 15 hospitals in Fujian, Zhejiang, and other places from July 1, 2018 to March 31, 2019. The inclusion criteria were the following: patients with chronic schizophrenia clinically diagnosed according to The Diagnostic and Statistical Manual of Mental Disorders-V, DSM-V (6) and patients who had volunteered for antipsychotic treatment but had not yet met the criteria for symptom relief. The exclusion criteria were the following: patients with severe hepatic and renal insufficiency, patients with severe allergic reactions and side effects treated with amisulpride, patients who failed to complete 8 weeks of drug treatment as required, follow-up dropouts, and cases with invalid research data. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The project was approved by the Medical Ethics Committee, and the informed consent was given by the enrolled patients and their families.
Therapies
All subjects were administered amisulpride tablets (Paco, Qilu Pharmaceutical Co., Ltd; approval no: SFDA approval no. H20113231; specification: 0.2 g*20 s) with a dose strategy of 200–1,200 mg/d for 8 weeks. The dose was adjusted according to the patient’s condition. If the daily dose of patients was less than or equal to 400 mg, the whole dose was taken once per day; if the daily dose exceeded 400 mg, it was taken in the morning and evening separately with an interval of least 8 h. In all cases, the maintenance dose was adjusted to the minimum effective dose based on the patient’s condition.
Evaluation index
- Efficacy evaluation (7,8): the therapeutic effect was evaluated by The Positive and Negative Affect Scale-6 (PANAS-6). Criterion A for symptom relief was defined as achieving a score of lower than or equal to 3 points for each of the 6 items. Criterion B was defined as achieving a total PANAS-6 score of lower than 14 points. After the course of treatment, the numbers of patients conforming to each of criteria A and B were counted.
- The improvement of depression symptoms was compared between the symptom remission group and the non-remission group. Depression symptoms were evaluated by the Calgary Depression Scale for Schizophrenia (CDSS) (9), which included a total of 9 items with 0 to 3 points for each item. Higher scores indicated more serious depression.
- Basic information and treatment-related data of subjects were collected, and logistic regression analysis was used to investigate the predictors of symptom relief in patients with schizophrenia treated with amisulpride.
Statistical methods
Statistical software SPSS 22.0 was used to process the data obtained from the study. The enumeration data are expressed as percentage (%),and χ2-test was used to analyze the difference between groups; the measurement data was expressed as mean ± criteria deviation (
Results
Efficacy analysis of amisulpride in the treatment of schizophrenia
Of the 383 patients with schizophrenia, 303 completed the 8-week treatment with amisulpride. Among these patients, 244 (80.53%) achieved remission of symptoms after treatment according to criterion A while 258 (85.15%) achieved remission according to criterion B. And no significant difference was found between the the number of patients who met criterion A and those who met criterion B (χ2=0.567, P=0.451). According to criterion A, there were 244 cases in the remission group and 59 cases in the non-remission group; according to criterion B, there were 258 cases in the remission group and 45 cases in the non-remission group. The 8-week efficacy of the remission group versus the non-remission group for criteria A and B is shown in Figure 1. The comparison between the remission group and the non-remission group in criteria A and B is shown in Figure 2.
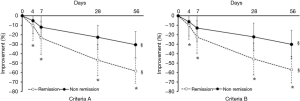
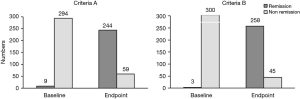
Comparison of data obtained from patients meeting criteria A and B
With reference to criteria A and B, Table 1 shows the demographic characteristics of the remission group versus the non-remission group of schizophrenia patients. Both groups showed significant improvement in depression symptoms, and the remission group showed more significant improvement in depression symptoms after treatment (P<0.05). The analysis of clinical data of patients during treatment in the remission group and the non-remission group according to criteria A and B is shown in Tables 2,3, respectively. Based on criterion A, there was no statistically significant difference between the data of patients in these two groups (P>0.05). However, based on criterion B, there were statistically significant differences in age, CDSS score, and some other aspects between the remission group and the non-remission group (P<0.05).
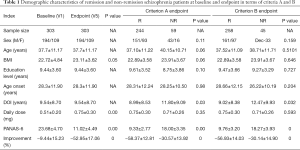
Full table

Full table
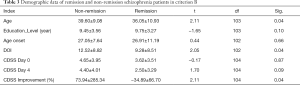
Full table
Analysis of predictors for remission of schizophrenia symptoms
Logistic regression analysis showed that, according to criterion A, the improvement of depression symptoms could not be used as a predictor for symptom relief in the treatment of schizophrenia with amisulpride (B=−0.01, P=0.07);according to criteria B, the improvement of depression symptoms did not predict the remission of schizophrenia symptoms, while the patient’s age could be used as a predictor for the remission of schizophrenia symptoms treated with amisulpride (B=−0.08, P=0.03). Each additional year of age, after other variables were controlled, was associated with a 7% reduction in symptom relief rate in schizophrenics. The results are shown in Tables 4,5.
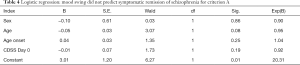
Full table
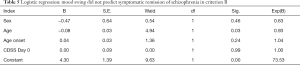
Full table
Discussion
Schizophrenia is a group of major mental diseases with unknown etiology, most of which have slow or sub acute onset in young adults (10). The prevalence rate of this disease is high, up to 6.55‰ according to domestic surveys in China, accounting for about half of the lifetime prevalence rate (13.70‰) of mental disorders except neuropathy. The disease not only seriously damages the patient’s mental and physical health, but also incurs a heavy burden on the patient’s family and surrounding society. This study was aimed to explore whether the improvement of depression symptoms is a predictive factor for the amelioration of the schizophrenia symptoms by amisulpride treatment. A total of 383 patients with schizophrenia were included in the study. These subjects were treated with amisulpride tablets with a dose strategy of 200–1,200 mg/d for 8 weeks. PANAS was used as the criteria to evaluate the treatment effect. The improvement of depression symptoms was compared between the remission group and the non-remission group. Logistic regression analysis was used to investigate predictors of symptom relief in schizophrenia patients treated with amisulpride.
Schizophrenia is a complicated disease in terms of both biology and methodology, and its etiology, pathogenesis, treatment, and prevention have always been a central subject in psychiatric research (11). As far as schizophrenia is concerned, clinically, it is believed to be caused by the comprehensive action of physiological, psychological, environmental, and other factors. Drug treatment is the first choice for the control of schizophrenia symptoms (12,13). In principle, for patients with mixed positive and negative symptoms, the initial treatment mainly aims to control the positive symptoms, while maintenance treatment mainly aims to control the negative symptoms. Amisulpride tablets are used to treat acute or chronic schizophrenia with positive symptoms (such as delirium, hallucination, cognitive impairment) and/or negative symptoms (such as delayed response, emotional apathy, and social withdrawal), as well as schizophrenia characterized by negative symptoms (14,15). Compared with the commonly used antipsychotic drug haloperidol, amisulpride can significantly improve the secondary negative symptoms of patients (16,17).
In this study, The results showed that 244 (80.53%) and 258 (85.15%) patients achieved remission of symptoms after treatment, reaching criteria A and B, respectively, indicating that amisulpride has a desirable effect on the treatment of schizophrenia. The comparison of the PANAS-6 scores between the remission group and the non-remission group, and the changes of CDSS scores in the remission group under criteria A and B, all indicated that the depression symptoms of the patients were improved to different degrees after amisulpride treatment. Some scholars have investigated the predictive value of early improvement of depression symptoms in schizophrenic symptom recovery of schizophrenics after treatment with quetiapine, believing that early improvement of depression symptoms can predict whether schizophrenic symptom recovery would be achieved after treatment with quetiapine in schizophrenia (18). However, in this study, according to criteria A and B of symptom remission, the improvement of depression symptoms could not be used as a predictor of symptom remission of schizophrenia, which was inconsistent with the above study. Since the sample included in this study was much larger than the 75 cases in the above-mentioned study, the results of this study might be more accurate. In the treatment of patients with schizophrenia, most patients have depression symptoms, and the side effects of antipsychotic drugs may increase the risk of depression; thus, the primary depression symptoms and secondary depression symptoms cannot be accurately distinguished, even though the treatment of amisulpride finally effectively improved the depression symptoms in terms of the overall course of treatment (19). In addition, the improvement of depressions symptoms and schizophrenic symptoms are also largely influenced by individual differences of patients, resulting in an inaccurate prediction of symptom remission in schizophrenics (20). In criterion B, logistic analysis showed that the patient’s age could be used as a predictor of the symptoms’ remission in the treatment of schizophrenia with amisulpride (B=−0.08, P=0.03). Specifically, for every 1-year increase in the age of a patient after controlling for other variables, the risk of symptoms remission of schizophrenia decreased by 7%. The result indicated that the therapeutic effect will decrease with the increase of patients’ age, which may be due to the degenerative changes of the patients’ body function with the increase of patient’s age, or due to the decrease of drug treatment tolerance and sensitivity, resulting in poor efficacy, which reflected the significance of early treatment for schizophrenia.
There are some limitations in this study, firstly the number size enrolled is small, secondly, long-time follow-up is lacking. All these may cause the statistical bias of this study. In conclusion, amisulpride has a remarkable effect on the treatment of schizophrenia and depression symptoms. The improvement of depression symptoms had no significant predictive value on the remission of schizophrenic symptoms.
Acknowledgments
Funding: General project of medical and health research in Zhejiang Province (2018KY879).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1702
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1702
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1702). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The project was approved by the Medical Ethics Committee, and the informed consent was given by the enrolled patients and their families.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fond G, Boyer L, Berna F, et al. Remission of depression in patients with schizophrenia and comorbid major depressive disorder: results from the FACE-SZ cohort. Br J Psychiatry 2018;213:464-70. [Crossref] [PubMed]
- Morozova M, Burminskiy D, Rupchev G, et al. 5-HT6 Receptor Antagonist as an Adjunct Treatment Targeting Residual Symptoms in Patients With Schizophrenia: Unexpected Sex-Related Effects (Double-Blind Placebo-Controlled Trial). J Clin Psychopharmacol 2017;37:169-75. [Crossref] [PubMed]
- Fountoulakis KN, Popovic D, Mosheva M, et al. Mood Symptoms in Stabilized Patients with Schizophrenia: A Bipolar Type with Predominant Psychotic Features? Psychiatr Danub 2017;29:148-54. [Crossref] [PubMed]
- Lysaker PH, Pattison ML, Leonhardt BL, et al. Insight in schizophrenia spectrum disorders: relationship with behavior, mood and perceived quality of life, underlying causes and emerging treatments. World Psychiatry 2018;17:12-23. [Crossref] [PubMed]
- Khanjani Z, Azmoodeh S, Mahmoudaliloo M, et al. A Comparison of Autistic Like Traits in the Relatives of Patients with Autism and Schizophrenia Spectrum Disorder. Iran J Psychiatry 2018;13:148-53. [PubMed]
- He H, Chang Q, Ma Y. The Association of Insight and Change in Insight with Clinical Symptoms in Depressed Inpatients. Shanghai Arch Psychiatry 2018;30:110-8. [PubMed]
- Manderbacka K, Arffman M, Suvisaari J, et al. Cancer care and mortality in patients with schizophrenia, substance use and mood disorders in Finland. Euro J Pub Health 2017;27:S79. [Crossref]
- Marshe V, Maciukiewicz M, Rej S, et al. Genome-Wide Association Study of Venlafaxine Treatment Remission in Late-Life Depression. Biological Psychiatry 2017;81:S95-6. [Crossref]
- Teixeira ML, Kuchiishi SS, Brandelli A, et al. Isolation of Haemophilusparasuis from diagnostic samples in the South of Brazil. Brazilian J Veter Pathol 2017;4:456-8.
- Swain SP, Behura SS, Dash MK, et al. The Influence of Psychosocial Dysfunctions in Chronic Schizophrenia Patients in Remission: A Hospital-Based Study. Indian J Psychol Med 2017;39:157. [Crossref] [PubMed]
- Cheon Y, Moon E, Park JM, et al. Can Residual Symptoms During Inter-Episode Period after Partial Remission in Bipolar I Disorder Have Cyclic Patterns with Specific Frequencies? Psychiatry Investig 2018;15:330-4. [Crossref] [PubMed]
- Girardi P, Casale AD, Rapinesi C, et al. Predictive factors of overall functioning improvement in patients with chronic schizophrenia and schizoaffective disorder treated with paliperidone palmitate and aripiprazole monohydrate. Hum Psychopharmacol 2018;33:e2658. [Crossref] [PubMed]
- Abraham KR., Kulhara PJ. The efficacy of electroconvulsive therapy in the treatment of schizophrenia. A comparative study. Br J Psychiatry 1987;151:152-5. [Crossref] [PubMed]
- Morimoto T, Matsuda Y, Matsuoka K, et al. Computer-assisted cognitive remediation therapy increases hippocampal volume in patients with schizophrenia: A randomized controlled trial. BMC Psychiatry 2018;18:83. [Crossref] [PubMed]
- Beebe L, Smith KD, Oppizzi L, et al. Telephone Intervention-Problem Solving (TIPS) for Schizophrenia Spectrum Disorders: Responses of Stable Outpatients Over Nine Months. Issues Ment Health Nurs 2018;39:561-7. [Crossref] [PubMed]
- Chiang ST, Lan CC. Quetiapine Related Acute Paralytic Ileus in a Bipolar I Disorder Patient with Successful Low Dose Amisulpride Substitution: A Case Report. Clin Psychopharmacol Neurosci 2018;16:228-31. [Crossref] [PubMed]
- McKenna P, Leucht S, Jauhar S, et al. The controversy about cognitive behavioural therapy for schizophrenia. World Psychiatry 2019;18:235-6. [Crossref] [PubMed]
- Kranke P, Bergese SD, Minkowitz HS, et al. Amisulpride Prevents Postoperative Nausea and Vomiting in Patients at High Risk: A Randomized, Double-blind, Placebo-controlled Trial. Anesthesiology 2018;128:1099-106. [Crossref] [PubMed]
- Raveendranthan D, Rao NP, Rao MG, et al. Add-on Aripiprazole for Atypical Antipsychotic-induced, Clinically Significant Hyperprolactinemia. Indian J Psychol Med 2018;40:38-40. [Crossref] [PubMed]
- Hashimoto K. Recent Advances in the Early Intervention in Schizophrenia: Future Direction from Preclinical Findings. Curr Psychiatry Rep 2019;21:75. [Crossref] [PubMed]
(English Language Editor: J. Gray)


