
Environmental exposure to cooking oil fumes and fatty liver disease
Introduction
Fatty liver disease (FLD) is characterized by fat deposits in liver cells and encompasses a broad array of liver pathology, ranging from asymptomatic steatosis to steatohepatitis, fibrosis, and cirrhosis (1). The economic burden of FLD on global health care systems is heavy (1). Along with lifestyle changes, the prevalence of FLD in has grown sharply in over recent years, with the mean prevalence in China reaching 15% in 2009 (2). Thus, it is important to seek out the related risk factors of FLD and effective preventative strategies.
Cooking is an essential part of daily life. Chinese-style cooking includes stir-frying and deep-frying, which require about 25–100 mL of cooking oil preheated to approximately 280 °C, which produces large quantities of cooking oil fumes (3). Cooking oil fumes contain an abundance of carcinogens, including polycyclic aromatic hydrocarbons (PAH), and fine particulate matters (PM), among others (4,5). It was reported that the total annual emission rate of PAH was 2,038 kg/year in Chinese restaurants, which is approximately 8 times higher than that in restaurants of western style cuisine (6). Another study reported that the proportion of PM2.5 emissions from cooking fuel was ~12% of the total global PM2.5 emissions (7). Several epidemiological studies have related cooking oil fumes to lung cancer and cardiovascular disease (8,9). Recently, Hou et al. found that environmental exposure to cooking oil fumes is also associated with diabetes (10). Few studies have analyzed the association between cooking oil fumes and FLD, despite the Ames test and SOS chromotest having found that cooking oil fumes contain genotoxic chemicals which are related to fatty deposition (11,12).
Therefore, the present study was conducted to investigate whether exposure to cooking oil fumes is related to the incidence and severity of FLD. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1730).
Methods
Participants
We performed a cross-sectional study based on the Ningbo database of the Cancer Screening Program in Urban China (CanSPUC) (13). The CanSPUC is an ongoing national cancer screening program which was initiated in October 2012. After providing signed written informed consent, all the eligible participants were interviewed face-to-face by trained staff in order to collect information about their exposure to risk factors. The cancer screening program in Ningbo, Zhejiang began in 2013, and a total of 55,959 participants (male =24,961, female =30,998) aged 40–75 years were included from 2013-2017. Participants with incomplete data on cooking oil fumes (n=1), FLD (n=899), and menopausal status (n=1) were excluded. Finally, 55,058 participants (male =24,551, female =30,507) were included in our analyses.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Cancer Hospital at the Chinese Academy of Medical Science (PJ-NBEY-KY-2020-144-01).
Questionnaires
Information regarding FLD, environmental exposure to cooking oil fumes, gender, age, menopausal status, weight, height, waist circumference, level of education, active and passive smoking, consumption of alcohol, dietary fat intake, regular exercise, and metabolic disease (hypertension, hyperlipidemia, and type II diabetes) were collected through face-to-face interviews. Participants who had self-reported FLD were further grouped as light fatty liver, moderate fatty liver, and heavy fatty liver by their responses to the question “how severe is the disease?”. Cooking oil fume exposure was graded as fumeless, light, moderate, and heavy. Body mass index (BMI) was calculated by dividing body weight (kg) by height (m2). Education was classified as illiteracy, primary or middle school, high school, and college or above. Information on smoking was obtained by enquiring about past and current smoking habits and subsequent classification as smoker (current or former smoker) or nonsmoker was made. Passive smoking was identified by asking whether there were smokers living in the participants’ family home or at their workplace. Alcohol consumption was classified as never, regular, and quit. Dietary fat intake was graded as high fat diet, moderate fat diet, and low-fat diet. Regular exercise was defined as the participants undertaking exercise ≥3 times a week and 30 min each time.
Statistical analysis
The characteristics of the participants were summarized as mean ± standard deviation (SD) for continuous variables and as frequency (%) for the categorical variables. Analyses of variance (ANOVA) and the Bonferroni test were used to compare continuous variables, and chi-squared (χ2) tests were used to compare categorical variables across the different cooking oil fume exposure groups.
Multiple logistic regression analyses were conducted to investigate the association between cooking oil fume exposure and FLD risk after adjusting for gender, age, BMI, waist circumference, level of education, active and passive smoking, alcohol consumption, dietary fat intake, regular exercise, and metabolic disease.
Interactive analysis was used to evaluate the interaction between cooking oil fume exposure and gender. A significant interaction was observed, and participants were then divided into 2 groups (male and female). Multiple logistic regression models were respectively rerun in males and female groups, and models in females were additionally adjusted for menopausal status. Furthermore, multiple ordinal logistic regression analyses were used to examine the association between cooking oil fume exposure and the severity of FLD with the same confounders adjusted as in the multiple logistic regression analyses (P value of proportional odds assumption <0.001). A value of P<0.05 (two sided) was considered statistically significant unless otherwise indicated. Stata version 13 was used for data analyses (Stata Corp., College Station, TX, USA).
Results
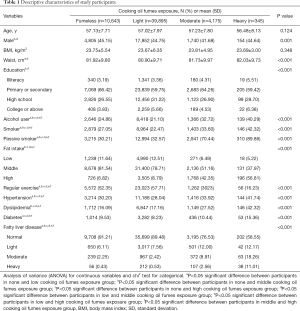
The characteristics of participants are shown in Table 1. The participants exposed to heavier cooking oil fumes were more likely to develop FLD (all P<0.001). Significant differences were found in waist circumference, education level, alcohol consumption, active and passive smoking, dietary fat intake, and regular exercise (all P<0.001). Participants in the heavy cooking oil fume exposure group had the highest risk of metabolic disease (hypertension, dyslipidemia, and type II diabetes, all P<0.05). There were no significant differences in age, menopausal status, and waist circumference across the cooking oil fume exposure groups (P>0.05).
Full table
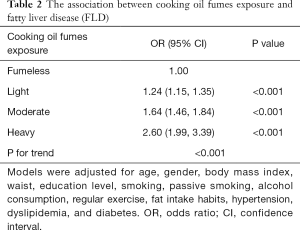
Associations between cooking oil fume exposure and prevalence of FLD are shown in Table 2. Compared with participants in the fumeless group, the adjusted odds ratios (ORs) for FLD among participants in light, moderate, and heavy cooking oil fume exposure groups were 1.24 (95% CI: 1.15–1.35, P<0.001), 1.64 (95% CI: 1.46–1.84, P<0.001), 2.60 (95% CI: 1.99–3.39, P<0.001), respectively. Participants with heavier cooking oil fume exposure tended to have higher risk of FLD (P<0.001).
Full table
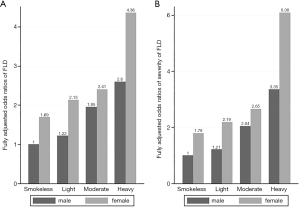
In the interactive analysis, we observed a significant interaction between cooking oil fume exposure and gender on the risk of FLD (OR for interaction =0.858, P for interaction =0.001). Compared with males in the fumeless group, females in the heavy cooking oil fume exposure group had the highest risk of FLD and the most severe disease extent among all participants (OR =4.36, P<0.001, Figure 1A; OR =6.08, P<0.001, Figure 1B).
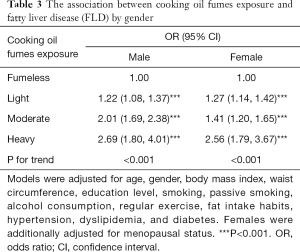
Thus, models were rerun with the participants divided into 2 groups according to gender (Table 3). Compared to participants in the fumeless group, males and females in light, moderate, and heavy cooking oil fume exposure groups all had significantly higher risk of FLD, while participants with heavier cooking oil fume exposure tended to have a higher risk of FLD (all P<0.001).
Full table
Associations between cooking oil fume exposure and severity of FLD were further analyzed in males and females, respectively (Table 4). Compared to participants in the fumeless group, males and females in light, moderate, and heavy cooking oil fume exposure groups all had significantly higher risk of more severe FLD (all P<0.001). Additionally, participants with heavier cooking oil fume exposure tended to have a higher risk of more severe FLD.
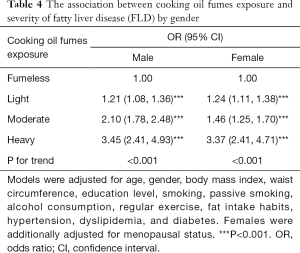
Full table
Discussion
In the present study, we found a positive association between cooking oil fume exposure and FLD after adjusting for various potential confounders in Chinese adults. Participants who were exposed to heavier cooking oil fumes tended to have a higher risk of FLD and more severe extent of disease. In addition, we observed a significant interaction between cooking oil fume exposure and gender on the risk of FLD. Female participants with heavy exposure to cooking oil fumes were associated with the highest observed OR of FLD and the most severe disease extent.
Cooking oil fume exposure has been found to be related with many chronic diseases such as lung cancer, cardiovascular disease, and diabetes (8-10). Previous animal studies have also found that the carcinogens contained in cigarette smoke, which can also be found in cooking oil fumes, might accelerate the deposition of fat in the liver (14). However, clinical data on the association between environmental exposure to cooking oil fumes and FLD is limited. In this study, we firstly confirmed that cooking oil fumes are significantly associated with FLD and more severe extent of disease, even after adjusting for possible confounders. In addition, participants with heavier cooking oil fume exposure tended to have higher ORs of FLD and more severe disease extent, suggesting a dose-response relationship between cooking oil fume exposure and FLD. We further adjusted diseases related to cooking oil fume exposure, including hypertension, dyslipidemia, and diabetes in our analyze and found an independent association between cooking oil fumes exposure and fatty liver disease. However, the mechanism underlying this association remains unclear. Plenty of evidence has shown that exposure to environmental PAHs induced oxidative DNA damage and elevated a systemic inflammatory marker (C-reactive protein) in the general population (15,16). Both DNA damage and the inflammatory response contribute to the decomposition of fatty acids, which is involved in the development of FLD (17,18). Similarly, PM, another important component of cooking oil fumes, has also been related to fat redistribution and metabolism (19,20). Additionally, Tan et al. found that PM that reached the liver could induce Kupffer cell cytokine secretion, which then triggered inflammation and initiated hepatic stellate cells to synthesize collagen (21).
In the interaction analyses, we found a synergistic effect between heavy cooking oil fume exposure and the female gender on FLD and more severe disease extent. Female, with heavy cooking oil fume exposure was associated with the highest risk for FLD and the most severe extent of disease, suggesting that females were more likely to be affected by cooking oil fumes than males. Consistent with our observations, previous studies have reported that females were more susceptible to environmental pollutants in relation to oxidative stress and diabetes than males (22,23). A possible explanation is the higher activity of cytochrome P450 1A1 (CYP1A1) which can produce higher levels of DNA adducts and lower the DNA repair capacity in females compared to males (24). In addition, body fat mass and abdominal fat mass have been documented to be associated with increased systemic inflammation, and reduced anti-inflammatory function, and may also have modifying effects on the cross-sectional association between environmental pollutants and metabolic disease (25). Thus, the different associations between cooking oil fumes and FLD among males and females might partly due to females generally having higher body fat mass and abdominal fat mass than males (26). However, in the stratified analysis, the associations between cooking oil fume exposure and FLD were found in both males and females. These results indicated that the positive association between exposure to cooking oil fumes and FLD should be noted in both males and females.
The present study had several strengths. Our study firstly related cooking oil fumes to FLD and found a dose-response relationship between exposure to cooking oil fumes and FLD. Secondly, the sample size was relatively large, making the statistical significance considerably robust. Thirdly, a series of important confounders, such as dietary fat intake, socio-economic factors, and metabolic disease, were taken into consideration in the analysis. Lastly, collapsing the environmental exposure of cooking oil fumes, and severity of FLD into 4 separate categories enabled us to have a better understanding on the associations between cooking oil fumes and FLD.
Conversely, limitations should also be noted. Firstly, because of the cross-sectional design, a causal relationship between cooking oil fumes and FLD may not be inferred. Secondly, the measurement of cooking oil fume exposure and FLD were based on self-report only, which may have led to recall bias and misclassification. Prospective and longitudinal studies should be performed in the future with more accurate information on cooking oil fume exposure and FLD. Thirdly, though several confounders have been adjusted in the present study, information on other potential confounders such as staying up late and overeating have not been collected. Fourthly, participants with nonalcoholic fatty liver disease (NAFLD) could not be distinguished from alcoholic fatty liver disease (AFLD) in our study. However, a sensitive analysis which had included only non-drinkers observed similar results (Tables S1,S2).
Conclusions
In conclusion, our study found that there may be a dose-dependent association between exposure to cooking oil fumes and FLD. In addition, heavy cooking oil fume exposure might have a synergistic effect with the female gender on FLD. These findings add to the current understanding regarding FLD prevention. Future studies should be conducted to explore the underlying mechanism of this association and whether effective cooking oil fume control measures like fume extractors in home kitchens can reduce the risk of FLD.
Acknowledgments
We sincerely thank the National Cancer Center of China (NCC). We would also like to thank the staff, physicians, and all the members of the Cancer Screening Program in Ningbo, China for providing support in the filling of surveys. Lastly, we are grateful to all participants for taking part in this study.
Funding: This study was supported by the Key Laboratory of Diagnosis and Treatment of Digestive System Tumors of Zhejiang Province (Grant No. 2019E10020), and the Ningbo Clinical Research Center for Digestive System Tumors (Grant No. 2019A21003).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1730
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1730
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1730). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Cancer Hospital at the Chinese Academy of Medical Science (PJ-NBEY-KY-2020-144-01). All the eligible participants signed written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singal AK, Kamath PS. Acute on chronic liver failure in non-alcoholic fatty liver and alcohol associated liver disease. Transl Gastroenterol Hepatol 2019;4:74. [Crossref]
- Fan JG, Farrell G. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol 2009;50:204-10. [Crossref] [PubMed]
- Tung YH, Ko J, Liang Y, et al. Cooking oil fume-induced cytokine expression and oxidative stress in human lung epithelial cells. Environ Res 2001;87:47-54. [Crossref] [PubMed]
- Singh A, Chandrasekharan N, Kamal R, et al. Assessing hazardous risks of indoor airborne polycyclic aromatic hydrocarbons in the kitchen and its association with lung functions and urinary PAH metabolites in kitchen workers. Clin Chim Acta 2016;452:204-13. [Crossref] [PubMed]
- Sjaastad AK, Jorgensen R, Svendsen K. Exposure to polycyclic aromatic hydrocarbons (PAHs), mutagenic aldehydes and particulate matter during pan frying of beefsteak. Occup Environ Med 2010;67:228-32. [Crossref] [PubMed]
- Li CT, Lin Y, Lee W, et al. Emission of polycyclic aromatic hydrocarbons and their carcinogenic potencies from cooking sources to the urban atmosphere. Environ Health Perspect 2003;111:483-7. [Crossref] [PubMed]
- Chafe ZA, Brauer M, Klimont Z, et al. Household cooking with solid fuels contributes to ambient PM2.5 air pollution and the burden of disease. Environ Health Perspect 2014;122:1314-20. [Crossref] [PubMed]
- Lin H, Tao J, Du Y, et al. Particle size and chemical constituents of ambient particulate pollution associated with cardiovascular mortality in Guangzhou, China. Environ Pollut 2016;208:758-66. [Crossref] [PubMed]
- Xia Z, Duan X, Tao S, et al. Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Environ Pollut 2013;173:150-6. [Crossref] [PubMed]
- Hou J, Sun H, Zhou Y, et al. Environmental exposure to polycyclic aromatic hydrocarbons, kitchen ventilation, fractional exhaled nitric oxide, and risk of diabetes among Chinese females. Indoor Air 2018;28:383-93. [Crossref] [PubMed]
- Chiang TA, Wu P, Ko Y. Identification of carcinogens in cooking oil fumes. Environ Res 1999;81:18-22. [Crossref] [PubMed]
- Chiang TA, Wu P, Wang L, et al. Mutagenicity and polycyclic aromatic hydrocarbon content of fumes from heated cooking oils produced in Taiwan. Mutat Res 1997;381:157-61. [Crossref] [PubMed]
- Chen H, Li N, Ren J, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut 2019;68:1450-7. [Crossref] [PubMed]
- Azzalini L, Ferrer E, Ramalho L, et al. Cigarette smoking exacerbates nonalcoholic fatty liver disease in obese rats. Hepatology 2010;51:1567-76. [Crossref] [PubMed]
- Kuang D, Zhang W, Deng Q, et al. Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to DNA and lipid in coke oven workers. Environ Sci Technol 2013;47:7446-56. [Crossref] [PubMed]
- Dung CH, Wu S, Yen G. Genotoxicity and oxidative stress of the mutagenic compounds formed in fumes of heated soybean oil, sunflower oil and lard. Toxicol In Vitro 2006;20:439-47. [Crossref] [PubMed]
- Akazawa Y, Nakashima R, Matsuda K, et al. Detection of DNA damage response in nonalcoholic fatty liver disease via p53-binding protein 1 nuclear expression. Mod Pathol 2019;32:997-1007. [Crossref] [PubMed]
- Prussick RB, Miele L. Nonalcoholic fatty liver disease in patients with psoriasis: a consequence of systemic inflammatory burden? Br J Dermatol 2018;179:16-29. [Crossref] [PubMed]
- Lawal AO. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol Lett 2017;270:88-95. [Crossref] [PubMed]
- Rajkumar S, Young B, Clark M, et al. Household air pollution from biomass-burning cookstoves and metabolic syndrome, blood lipid concentrations, and waist circumference in Honduran women: A cross-sectional study. Environ Res 2019;170:46-55. [Crossref] [PubMed]
- Tan HH, Fiel M, Sun Q, et al. Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease. J Immunotoxicol 2009;6:266-75. [Crossref] [PubMed]
- Guo H, Huang K, Zhang X, et al. Women are more susceptible than men to oxidative stress and chromosome damage caused by polycyclic aromatic hydrocarbons exposure. Environ Mol Mutagen 2014;55:472-81. [Crossref] [PubMed]
- Hansen AB, Ravnskjaer L, Loft S, et al. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ Int 2016;91:243-50. [Crossref] [PubMed]
- Uppstad H, Osnes G, Cole K, et al. Sex differences in susceptibility to PAHs is an intrinsic property of human lung adenocarcinoma cells. Lung Cancer 2011;71:264-70. [Crossref] [PubMed]
- Kim HJ, Kwon H, Jeong S, et al. Effects of abdominal visceral fat compared with those of subcutaneous fat on the association between PM10 and hypertension in Korean men: A cross-sectional study. Sci Rep 2019;9:5951. [Crossref] [PubMed]
- Yang M, Lin J, Ma X, et al. Truncal and leg fat associations with metabolic risk factors among Chinese adults. Asia Pac J Clin Nutr 2016;25:798-809. [PubMed]
(English Language Editor: J. Jones)


