
Mid-back pain due to a penetrating atherosclerotic aortic ulcer: do not miss the diagnosis—a case report
Introduction
Although mid-back pain is less common than neck and low back pain, pain physicians frequently meet patients who complain of mid-back pain (1,2). The 1-year prevalence of mid-back pain was reported to be approximately 15% (1,2). When pain physicians encounter patients with mid-back pain, they initially consider the pain to be of musculoskeletal in origin, such as thoracic facet or thoracic disc pathologies, thoracic radiculopathy, or myofascial pain syndrome (3-5). However, several disorders other than lesions in the musculoskeletal system, such as PAU, aortic dissection or aneurysm, gastric ulcer, kidney stone, pancreatitis, and abdominal malignancy, can also cause mid-back pain. Therefore, prior to confirming the diagnosis of musculoskeletal system pathologies, the presence of other internal organ disorders should be closely considered.
PAU is defined as an atherosclerotic plaque ulceration that penetrates the intima then progresses into the tunica media layer with or without associated pseudoaneurysm or intramural hematoma (6). Patients with PAU can have back or chest pain (7,8). However, the diagnosis is often delayed owing to its low incidence.
In this study, we report a patient with mid-back pain of which the cause was eventually found to be a PAU, after several procedures for the purpose of diagnosing the cause of pain and reducing pain in the pain clinic.
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1568).
Case presentation
A 65-year-old man visited the pain clinic in our university hospital for mid-back pain [numeric rating scale (NRS): 7; 0: no pain, 10: most intense pain imaginable] sustained for 2 months (Figure 1). His pain was aggravated in the supine and sitting positions. After approximately 1 min in the supine position, his mid-back pain was initialed at the Lt. paraspinal area (T9–T11 vertebral levels) and radiated to the left lateral chest. Furthermore, the mid-back pain appeared after sitting for approximately 30 min. The patient’s pain was relieved in the decubitus and standing up-right positions. He had a past medical history of hypertension and was taking amlodipine at home. Upon chest X-ray, no abnormality was found. One month earlier, the patient had undergone coronary angiography for pain assessment, but the findings were normal. Electrocardiogram, serum troponin I, & CK-MB were also normal. The possible causes of cardiac-related pain were ruled out. Moreover, abdominal computed tomography (CT) revealed no abnormality. In the X-ray and magnetic resonance imaging (MRI) of the cervical and thoracic spine, no specific abnormal findings were observed. On physical examination, no motor or sensory deficit was observed. In addition, deep tendon reflexes (knee and ankle jerks) were normal. Tenderness was checked on around the Lt. T9–T10 and T10–T11 thoracic facet joints. In the prone position, when the Lt. T9–T10 and T10–T11 facet joint areas were pushed by the examiner’s hands, his usual pain occurred. Oral pain medications (tramadol hydrochloride 75 mg per day) and trigger point injection on the Lt. thoracic paraspinalis muscles with 3 mL of 2% lidocaine and 5 mL of normal saline did not show efficacy. We conducted a fluoroscopy-guided medial branch injection on the Lt. T8, 9, 10, and 11 with a mixed solution of 0.5 mL of 2% lidocaine, 0.5 mL of 0.25% bupivacaine, and 5 mg of dexamethasone with the diagnostic and therapeutic purpose of thoracic facet joint pain. At the 2-week follow-up after the medial branch injection, the patient reported that the pain seemed to have decreased for 2 h after the procedure (but not definite); however, after 2 h, the pain returned to the same level felt prior to the procedure. We conducted pulsed radiofrequency stimulation (Cosman G4 radiofrequency generator, Cosman Medical, USA) on the Lt. T8, 9, 10, and T11 medial branches (5 Hz and 5 ms pulse width for 360 s at 45 V) under fluoroscopy (9). However, any effectiveness was not manifested.

Approximately 2 weeks after the pulsed radiofrequency stimulation (3 months after his symptom onset), the patient visited the emergency room for aggravated Lt. side mid-back pain (NRS: 8). A high blood pressure (147/81 mmHg) was measured. However, the respiratory rate (20 breaths/min), pulse rate (98 beats/min), and body temperature (36.6 °C) were within normal ranges. A 3-dimensional CT aortography was performed, revealing intraluminal thrombus, multiple ruptured and unruptured PAUs (largest transverse diameter, 8 mm; largest depth, 6 mm), and aneurysmal change of the descending thoracic aorta (Figures 2-4). Any abnormalities in the heart, abdominal organs, or thoracic spine and muscles were not detected. Accordingly, the PAU was diagnosed as a source of the patient’s pain. Accordingly, we administered nicardipine with a rate of 1.15 mcg/kg/min and esmolol with a rate of 100 mcg/kg/min for controlling the systolic blood pressure. In addition, an anticoagulant (tranexamic acid 750 mg/day) was administered orally. To alleviate the pain, we further administered intravenous opioid (remifentanil hydrochloride 10 mg/day). Approximately 6 h later, the systolic blood pressure decreased to 100–120 mmHg, and the pain rating decreased to NRS 1. One week after the admission, the 3-dimensional CT aortography result was followed up, and any changes of aortic lesions were not found. The patient’s pain almost completely disappeared. We changed the intravenous antihypertensive medications to oral drugs (amlodipine besylate 10 mg/day, bisoprolol fumarate 5 mg/day, candesartan cilexetil/hydrochlorothiazide 16/12.5 mg/day, and acetaminophen/codeine phosphate/ibuprofen 750/30/600 mg/day). At the 2-week follow-up after the discharge, blood pressure was measured to be 110/70 mmHg and the pain was completely disappeared.
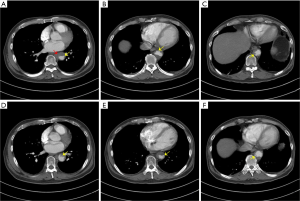
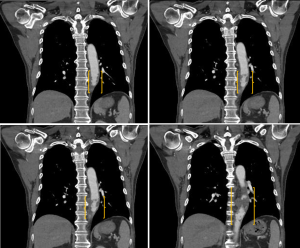
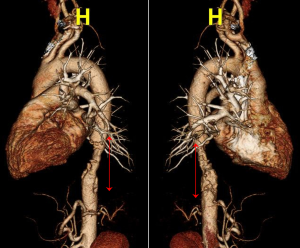
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Here, we reported a patient who had mid-back and lateral chest pain due to multiple PAUs in the descending thoracic aorta. Initially, we considered that the patient’s pain was associated with musculoskeletal disorders, such as thoracic facet pathology or myofascial pain syndrome in the thoracic paraspinal muscle. However, several procedures for musculoskeletal pain reduction were ineffective. After the PAU diagnosis, we administered antihypertensive medications combined with analgesics to effectively control the patient’s pain.
Myofascial pain syndrome and thoracic facet joint origin pain are the most frequent causes of mid-back pain. Moreover, thoracic facet origin pain can be occasionally referred to the lateral and anterior chest areas (10). If the thoracic MRI does not reveal thoracic intervertebral disc herniation or thoracic spine fracture, pain physicians believe that the most possible cause of mid-back pain would be myofascial pain syndrome on the paraspinal muscle and thoracic facet joint origin pain. However, in our case, we ruled out musculoskeletal problems through spine MRI and diagnostic/therapeutic injections. By performing a 3-dimensional CT aortography, we diagnosed the patient as having multiple PAUs in the descending thoracic aorta.
Although the incidence of PAU remains unknown, it is recognized as a rare disorder (8). Because the clinical presentation of PAU is frequently vague, PAU is commonly diagnosed late after the other pathologies which can cause mid-back or chest pain are ruled out (8). However, it can cause serious complications, such as aortic dissection, aortic rupture, and aortogastric fistula, which can lead to death (11-13). Therefore, the diagnosis of PAU should not be missed. In the previous study, 75% of patients who had PAU had mid-back or chest pain (14). In the early stage, aortic ulcer developed within the intima and often asymptomatic (15). With further progression of PAU, the ulcer developed in the medial layer and caused mid-back or chest pain (15). Also, it can lead to hematoma formation within the medial layer. The risk factors for PAU are atherosclerotic disease, coronary artery disease, hypertension, old age, hyperlipidemia, and smoking history (14,16). Additionally, it is predominantly involved in the middle and distal descending thoracic aorta (8). CT aortography is the most appropriate choice for the diagnosis of PAU (8,17,18). Also, MRI and transesophageal echocardiography can be helpful for the diagnosis of PAU (17,18). The typical finding of PAU is a contrast-filled, out-pouching of the aortic wall or into the thickened aortic wall in the absence of an intimal flap or a false lumen (17). In MRI, high intensity in the aortic wall can be found in the T1- and T2-weighted images (17). In transesophageal echocardiography, the finding of crater-like or focal out-pouching with rough edges in an atherosclerotic aortic wall is revealed (18). Regarding the treatment of PAU, medical treatment with antihypertensive agents is administered to patients with uncomplicated PAU, and endovascular or surgical repair is considered in complicated cases, including symptomatic patients despite medical treatment, asymptomatic patients with large pleural effusion, presence of intramural hematoma, and large PAU depth (>10 mm) and diameter (>20 mm) (19). Our patient showed good response to the medical treatment, and he had no indications for surgical treatment. Therefore, we sustained medical treatment for managing pain from PAU.
In conclusion, we reported a patient with mid-back and lateral chest pain, which was likely to be induced by PAU. Clinicians should consider the possibility of PAU in patients who complain of mid-back and chest pain, especially when the other pathologies that can cause those pains were not ruled out.
Acknowledgments
Funding: The present study was supported by a National Research Foundation of Korea grant funded by the Korean government (grant No. NRF-2019M3E5D1A02069399).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1568
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1568). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Linton SJ, Hellsing AL, Halldén K. A population-based study of spinal pain among 35-45-year-old individuals. Prevalence, sick leave, and health care use. Spine (Phila Pa 1976) 1998;23:1457-63. [Crossref] [PubMed]
- Manchikanti L, Singh V, Datta S, et al. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician 2009;12:E35-70. [Crossref] [PubMed]
- Botwin KP, Baskin M, Rao S. Adverse effects of fluoroscopically guided interlaminar thoracic epidural steroid injections. Am J Phys Med Rehabil 2006;85:14-23. [Crossref] [PubMed]
- Chen CK, Nizar AJ. Myofascial pain syndrome in chronic back pain patients. Korean J Pain 2011;24:100-4. [Crossref] [PubMed]
- Lee DG, Ahn SH, Cho YW, et al. Comparison of Intra-articular Thoracic Facet Joint Steroid Injection and Thoracic Medial Branch Block for the Management of Thoracic Facet Joint Pain. Spine (Phila Pa 1976) 2018;43:76-80. [Crossref] [PubMed]
- Sundt TM. Intramural hematoma and penetrating atherosclerotic ulcer of the aorta. Ann Thorac Surg 2007;83:S835-41. [Crossref] [PubMed]
- Cooke JP, Kazmier FJ, Orszulak TA. The penetrating aortic ulcer: Pathologic manifestations, diagnosis, and management. Mayo Clin Proc 1988;63:718-25. [Crossref] [PubMed]
- Kyaw H, Sadiq S, Chowdhury A, et al. An uncommon cause of chest pain - penetrating atherosclerotic aortic ulcer. J Community Hosp Intern Med Perspect 2016;6:31506. [Crossref] [PubMed]
- Chang MC. Effect of Pulsed Radiofrequency Treatment on the Thoracic Medial Branch for Managing Chronic Thoracic Facet Joint Pain Refractory to Medial Branch Block with Local Anesthetics. World Neurosurg 2018;111:e644-8. [Crossref] [PubMed]
- Dreyfuss P, Tibiletti C, Dreyer SJ. Thoracic zygapophyseal joint pain patterns. A study in normal volunteers. Spine (Phila Pa 1976) 1994;19:807-11. [Crossref] [PubMed]
- Brinster DR. Endovascular repair of the descending thoracic aorta for penetrating atherosclerotic ulcer disease. J Card Surg 2009;24:203-8. [Crossref] [PubMed]
- Dalio MB, Dezotti NR, Ribeiro MS, et al. Aortogastric fistula due to a penetrating atherosclerotic aortic ulcer. Ann Vasc Surg 2015;29:1659.e21-5. [Crossref] [PubMed]
- Stanson AW, Kazmier FJ, Hollier LH, et al. Penetrating atherosclerotic ulcers of the thoracic aorta: Natural history and clinicopathologic correlations. Ann Vasc Surg 1986;1:15-23. [Crossref] [PubMed]
- Cho KR, Stanson AW, Potter DD, et al. Penetrating atherosclerotic ulcer of the descending thoracic aorta and arch. J Thorac Cardiovasc Surg 2004;127:1393-9; discussion 1399-1401. [Crossref] [PubMed]
- Kotsis T, Spyropoulos BG, Asaloumidis N, et al. Penetrating Atherosclerotic Ulcers of the Abdominal Aorta: A Case Report and Review of the Literature. Vasc Specialist Int 2019;35:152-9. [Crossref] [PubMed]
- Patatas K, Shrivastava V, Ettles DF. Penetrating atherosclerotic ulcer of the aorta: A continuing debate. Clin Radiol 2013;68:753-9. [Crossref] [PubMed]
- Litmanovich D, Bankier AA, Cantin L, et al. CT and MRI in diseases of the aorta. AJR Am J Roentgenol 2009;193:928-40. [Crossref] [PubMed]
- Vilacosta I, San Roma’n JA, Aragoncillo P, et al. Penetrating atherosclerotic aortic ulcer: Documentation by transesophageal echocardiography. J Am Coll Cardiol 1998;32:83-9. [Crossref] [PubMed]
- Evangelista A, Czerny M, Nienaber C, et al. Interdisciplinary expert consensus on management of type B intramural haematoma and penetrating aortic ulcer. Eur J Cardiothorac Surg 2015;47:209-17. [Crossref] [PubMed]


