
A rare case of indolent B cell lymphoma with massive pleural effusion as the initial presentation
Introduction
Pleural effusion is a common lymphoma complication caused by pleural involvement, infection, and obstruction of lymphatic return or hypoproteinemia. It is indicative of poor prognosis, although it is rare as the primary occurrence and initial presentation in lymphoma cases (1,2). Primary pleural lymphoma is an extremely rare condition that accounts for only 0.3–1% of all extranodal lymphomas (3) and only affects pleura in the absence of a solid tumor. The two major subtypes of primary pleural lymphoma in immunocompromised patients are primary effusion lymphoma (PEL) and chronic pyothorax-associated lymphomas (PAL), characterized by large B-cell malignancies (4). However, it is extremely rare that indolent small B-cell lymphoma in primary pleural lymphoma. The case in the current study was an unusual presentation of pleural lymphoma because it occurred on an immunocompetent adult woman without HIV, HHV8, EBV or a history of chronic pyothorax, uncharacteristic of PEL and PAL.
A few case reports have identified other types of pleural lymphoma, such as extra-nodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (EMZL/MALT-type) and mantle cell lymphoma. Ben et al. (5) had reported a case of pleural lymphoma presenting with irregular pleural mass and pleural effusion, was confirmed as extranodal marginal zone B-cell lymphoma by histopathology. One case in Chinese literature (6) was primary pleural small B-cell lymphocytic lymphoma, diagnosed by pleural mass biopsy. Two cases of mantle cell lymphoma mainly involving thoracic lesions as the initial presentation had also been reported in previous studies (7). But it is unique in our case that the diagnosis was initial confirmed by flow cytometry of pleural effusion, which showed that the abnormal B cells were marginal zone B cell phenotype.
In this paper, we report a case of indolent B cell lymphoma, possibly marginal zone lymphoma, in an immunocompetent woman. The condition manifested as massive pleural effusion which had been misdiagnosed for 2 years. We describe the diagnosis process and review the clinical characteristics of this rare condition. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1480).
Case presentation
A 52-year-old female patient was admitted to our hospital on March 5, 2019, with a 2-year history of massive unexplained pleural effusion. More than two years ago, in August 2016, the patient had attended other hospital (China-Japan Union Hospital of Jilin University) with intermittent shortness of breath, without cough, fever and weight loss. In October, 2016, a computerized tomography (CT) scan revealed a bilateral pleural effusion with small patches on her right lung, with multiple drainages of pleural effusion indicating exudate effusion. In addition, in December 2016, percutaneous transthoracic needle lung biopsies showed inflammatory cell infiltration and fibrous tissue hyperplasia, whereas a right-side thoracoscopic pleural biopsy indicated proliferative fibrous tissue. No evidence of tumor, autoimmune disease, tuberculosis or infection was found. Between December, 2016 and November, 2018, the patient was put on 23 months of anti-tuberculosis medication and intermittent drainage of pleural effusion. From October 2016 to March 2019, a total of 75 liters of pleural effusion were drained, although the etiology of long-term massive pleural effusion remains unknown. Since she had a prior history of hypothyroidism, sodium L-thyroxine (50 ug/d) was prescribed. She was a lifelong non-smoker, with no known exposure to asbestos or other chemical agents. Physical examination on the patient revealed a normal vital signs. Her basilar breathing sounds were bilaterally reduced, with no finger clubbing, yellow nails, lymphadenopathy, or hepatosplenomegaly. The rest of her physical condition was normal.
Diagnostic studies
Diagnostic examinations after admission showed normal blood, liver, kidney and thyroid function, as well as profiles of autoantibodies and tumor markers. On March 6, 2019, radiography and ultrasound on her chest revealed a bilateral pleural effusion, and further indicated that the right-side pleural effusion had been encapsulated while the left-side was free. In addition, left-side thoracentesis which was examined on March 9, 2019, resulted in 80% lymphocytes. Pleural fluid confirmed exudative under Light’s criteria [serum protein 77 g/L and lactate dehydrogenase (LDH) 220 U/L; pleural protein 52 g/L and LDH 806 U/L]. Furthermore, results from serological analyses indicated no hepatitis C (HCV), human immunodeficiency virus (HIV), and human herpesvirus 8 (HHV-8) antibodies as well as Epstein-Barr virus (EBV) viral load. An extensive investigation for infectious agents of pleural effusion, including bacteria, tuberculosis, parasites revealed negative results. Similarly, testing of pleural effusion targeting HHV-8 nucleic acid also revealed negative results. On March 10, cytopathology of pleural effusion revealed numerous lymphocytes, with normal morphology but without atypia.
On March 12, 2019, we performed positron emission computed tomography (PET-CT) (Figure 1), after draining 1,800 mL of left-side pleural effusion, and confirmed the presence of bilateral pleural effusion. However, there was no evidence of pleural calcification or lymphadenopathy, although a few interstitial changes, patches and cords were observed in both lungs. PET-CT also revealed a right adnexal cyst, measuring 4.9×4.5×4.0 cm3, but without ascites.
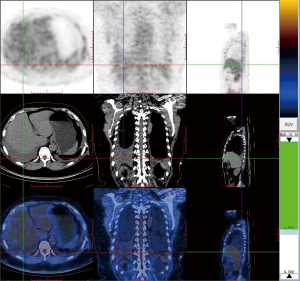
Despite absence of ascites, Meigs syndrome was considered the most likely etiology, because lymphocytic exudate pleural effusion coexists with adnexal cyst. On March 27, 2019, laparoscopic resection of adnexal cyst was performed. A final pathological result confirmed presence of a serous cystadenoma, a rare type for Meigs syndrome. However, postoperative reexamination indicated no decrease in pleural effusion.
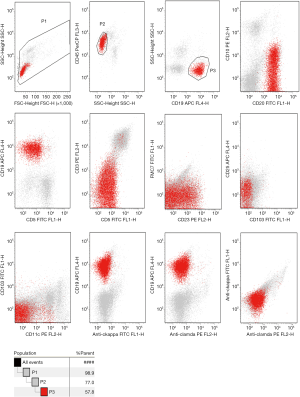
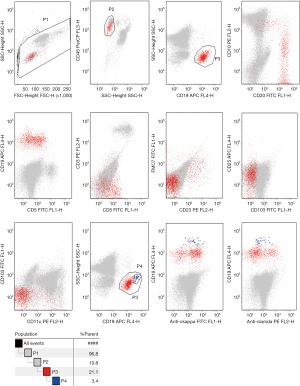
To examine hematological malignancies, we performed flow cytometry of pleural effusion on March 30, 2019, and found proliferation of B lymphocytes with abnormal immune markers, including CD19+ B cells (accounts for 57.8% of total lymphocytes), FSClow, CD19+, CD20+, CD10−, CD5−, FMC7−, CD23−/+, CD103−, CD25−, CD11c−/+, and no light chain restriction (Figure 2). On April 5, 2019, we performed bone marrow aspiration and biopsy further. Flow cytometry on bone marrow revealed ckappa restrictive expression on B cells (Figure 3). Lymphocytes constituted 10.8% of the nucleated cells, of which 21.1% were B cells, with the phenotype: FSClow, CD19+, CD20+, CD10−, CD5−, FMC-7−, CD23−/+, CD103−, CD25−, CD11c−/+, ckappa−, clamda−. In B cells, 3.4% cells were clonal B cells with abnormal phenotype: CD19hi, ckappa+, clamda−. Bone marrow smear and biopsy pathology were unremarkable. Immunocytochemistry, performed on pleural effusion’s cell block on April 8, 2019, showed massive small lymphocytes with BCL2++ and CD43++. Furthermore, no clonal heavy chain rearrangement was detected in pleural effusion and the bone marrow.
On April 15, 2019, a left-side video-assisted thoracoscopy revealed diffuse fiber separation of the pleural cavity, whereas pathological analysis of pleural cavity contents indicated degeneration of the fibrinoid with aggregation to the small B lymphocytes. On the other hand, pathology of parietal pleura indicated fibrinous pleurisy without tumor cell infiltration.
Overall, these findings revealed abnormal B cells proliferation in the patient’s pleural effusion and a clone of small B cells in bone marrow, with restricted ckappa expression. Characteristics of the B cells immune markers excluded chronic lymphocytic or hairy cell leukemia, as well as mantle cell or lymphoplasmacytic cell lymphoma, thereby supporting the probable existence of a marginal zone lymphoma.
Clinical course
The patient’s symptom relief and the left-side pleural fluid was also encapsulated after thoracoscopy. Considering the foreseeable side effects of chemotherapeutic drugs and indolent potential of the small B cell lymphoma, the patient opted not to undergo further treatment and discharged on April 24, 2019. Follow up was done 1, 3 and 6 months after discharge, with no more thoracentesis preformed. At the end, the depths of bilateral pleural effusion on ultrasound stabilized around 3 cm. The patient is feeling perfectly well, and has no shortness of breath. The whole course of diagnosis and treatment was organized as a timeline (Figure 4).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees(s) and with the Helsinki Declaration (as revised in 2013). All the data in this case report are reported with the informed consent of the patient.
Discussion
In this article, we report a rare case of small B cell lymphoma, manifested as massive pleural effusion. Although differential diagnosis was challenging, flow cytometry of pleural effusion revealed B-cells with abnormal immunophenotypes finally.
In the absence of any evidence of disease outside the pleural space, primary pleural lymphoma was considered. To date, studies have described two major types of primary pleural lymphoma; PEL and chronic PAL (8). PEL was first described in 1996 in HIV-infected individuals who were coinfected with Kaposi’s sarcoma-associated herpesvirus (KSHV) or HHV-8 virus. This subtype was also defined as a separate entity in World Health Organization’s classification of tumors occurring in hematopoietic and lymphoid tissue in 2001 (9). Although it rarely occurs, PEL has been reported in patients following solid organ transplantation, immunosuppression or Hepatitis C infection in HHV-8 negative (10). On the other hand, PAL is associated with a history of chronic pyothorax, or chronic inflammation of the pleura following previous artificial pneumothorax for treatment of tuberculosis. It has been hypothesized that long-standing pleural inflammation is a predisposing factor for this lymphoma, with a strong EBV association. PEL and PAL are both diffuse large B-cell lymphomas with poor prognosis (11). However, indolent small B-cell lymphoma in primary pleural lymphoma is extremely rare and has completely different characteristics compared with PEL or PAL.
Previous case studies have reported the identification of small B-cell pleural lymphomas such as marginal zone B-cell and mantle cell, in HIV and HHV-8-negative immunocompetent patients (6). Motta et al. (12) reviewed 8 cases of marginal zone lymphoma with primary pleural presentation, and reported that all of them appeared to be indolent after clinical course, which is different from PEL and PAL. Particularly, half of the patients achieved complete remission after local treatments, including pleural decortication/pleurodesis with or without chemotherapy. Previous studies have also reported that several patients who had not been treated, but were followed up for 6–40 months, exhibited no significant progress (13,14). Nevertheless, pathogenesis and disease outcomes of primary pleural marginal zone lymphoma are totally different from typical PEL and PAL.
In malignancy-related serous effusions, cytology remains the first-line, cost-effective and rapid diagnostic tool. However, cytomorphology alone cannot differentiate between reactive lymphocytosis and lymphoma in cases of low grade lymphomas or leukemias manifested as a small number of atypical cells (15). In addition, a low (30%) diagnostic yield can be expected from pleural fluid because these fluid specimens harbor degenerating lymphoid cells or cells indistinguishable from reactive lymphocytes. However, immunocytochemistry and phenotyping have helped enhance the yield by 8–16% (16). In the current study, although cytopathology of pleural effusion showed lymphocytes with normal morphology, flow cytometry identified abnormal B cells, which was confirmed by immunohistochemical of pleural effusion. Flow cytometry is a critical tool for routine diagnosis of hematopoietic neoplasms, especially in small B-cell lymphomas with normal morphology. It is particularly suited for cells in fluid specimens, where cell aggregates and extracellular matrix elements that may interfere with generating single cells for analysis, are less prominent compared to solid tissue samples (17). In addition, flow cytometry helps in early diagnosis of hematopoietic neoplasms associated with pleural effusion and is therefore recommended for identifying unexplained lymphocytic exudate pleural effusion. In our case, the process allowed detection of elevated B-cell levels as well as abnormal markers in the exudate, which did not support reactive pleural effusion dominated by T cells (18). However, ckappa restriction was not detected in pleural effusion, and was only present in the bone marrow. This could have been due to: first, the high protein content in pleural effusion could have interfered with detection of monoclonal light chain; second, disruption of the cell membrane may have resulted in cell loss, affecting the detection of clonal expression. Since the proportion of abnormal B cells was relatively low, no evidence of infiltration was found in the pleural tissue at this stage. However, the patient still requires further observation and close follow-up.
In this article, we reported a rare case of small B cell lymphoma only manifested as massive pleural effusion. The case was an unusual presentation because it occurred on an immunocompetent adult woman and the type of malignant lymphoma was small B cell lymphoma, which were totally different from PEL or PAL. To our knowledge, this is the first time that the diagnosis was confirmed by cytological evidence using flow cytometry of pleural effusion, in the absence of histopathology. This may provide a new idea for the diagnosis of unexplained pleural effusion. However, the case report has the following limitations. First, this is a case report of single patient and more cases will be needed to support it in the future. Second, at present, the follow-up time of this patient is relatively short, and a long time of follow-up is needed to observe the development of the disease.
In conclusion, small B cell lymphoma may only manifest as long-term massive pleural effusion, which is extremely rare and different from the two major types of primary pleural lymphoma. Flow cytometry can effectively diagnose unexplained pleural effusion, especially in small B-cell lymphomas with normal morphology.
Acknowledgments
We thank the staff members of department of geriatrics, hematology, thoracic surgery and gynecology in Peking University First Hospital for their assistance with the diagnosis process of this case.
Funding: Financial support for this work was received from the Scientific Research Seed Fund of Peking University First Hospital (2018SF058).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1480
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1480). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sukswai N, Lyapichev K, Khoury JD, et al. Diffuse large B-cell lymphoma variants: an update. Pathology 2020;52:53-67. [Crossref] [PubMed]
- Porcel JM, Cuadrat I, Garcia-Cerecedo T, et al. Pleural Effusions in Diffuse Large B-Cell Lymphoma: Clinical and Prognostic Significance. Lung 2019;197:47-51. [Crossref] [PubMed]
- Li A, Poon L, Khoo KL, et al. A man with pleural effusion and ascites. Chest 2015;147:e208-e214. [Crossref] [PubMed]
- Zanelli M, Bertuzzi C, Zizzo M, et al. Extracavitary primary effusion lymphoma in a post-transplantation patient. Br J Haematol 2019;187:555. [Crossref] [PubMed]
- Ben Saad A, Fahem N, Khemakhem R, et al. Rare case of primary extranodal marginal zone lymphoma of the thorax. Respir Med Case Rep 2019;26:251-4. [Crossref] [PubMed]
- Ru X, Ge M, Li L, et al. Primary pleural lymphoma: a rare case and a synopsis of the literature. J Thorac Dis 2013;5:E121-3. [PubMed]
- Kosaka M, Koizumi T, Fukushima T, et al. Mantle cell lymphoma mainly involving thoracic lesions: two case reports. Intern Med 2011;50:1477-81. [Crossref] [PubMed]
- Ahmad H, Pawade J, Falk S, et al. Primary pleural lymphomas. Thorax 2003;58:908-9. [Crossref] [PubMed]
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther 2018;11:3747-54. [Crossref] [PubMed]
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: Report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis 2019;21:e13005 [Crossref] [PubMed]
- Taniguchi A, Hashida Y, Nemoto Y, et al. Epstein-Barr Virus-Positive Pyothorax-Associated Lymphoma Arising from a Posttraumatic Empyema. Acta Haematol 2015;134:155-60. [Crossref] [PubMed]
- Motta G, Conticello C, Amato G, et al. Pleuric presentation of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue: a case report and a review of the literature. Int J Hematol 2010;92:369-73. [Crossref] [PubMed]
- Adiguzel C, Bozkurt SU, Kaygusuz I, et al. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: report of a rare case and review of the literature. Apmis 2009;117:222-9. [Crossref] [PubMed]
- Mitchell A, Meunier C, Ouellette D, et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue with initial presentation in the pleura. Chest 2006;129:791-4. [Crossref] [PubMed]
- Yu GH, Vergara N, Moore EM, et al. Use of flow cytometry in the diagnosis of lymphoproliferative disorders in fluid specimens. Diagn Cytopathol 2014;42:664-70. [Crossref] [PubMed]
- Savvidou K, Dimitrakopoulou A, Kafasi N, et al. Diagnostic role of cytology in serous effusions of patients with hematologic malignancies. Diagn Cytopathol 2019;47:404-11. [Crossref] [PubMed]
- Iqbal J, Liu T, Mapow B, et al. Importance of flow cytometric analysis of serous effusions in the diagnosis of hematopoietic neoplasms in patients with prior hematopoietic malignancies. Anal Quant Cytol Histol 2010;32:161-5. [PubMed]
- Goseva Z, Kaeva B, Gjorcev A, et al. Analysis of Lymphocyte Immunological Reactivity in Patients with Pleural Effusions of Different Aetiology. Open Access Maced J Med Sci 2016;4:50-3. [Crossref] [PubMed]



