Retrospective review of the incidence of monitoring blood glucose levels in patients receiving corticosteroids with systemic anti-cancer therapy
Introduction
Since their effectiveness as antiemetics was first shown 30 years ago, corticosteroids have been used in conjunction with chemotherapy to prevent side effects such as acute or delayed nausea and vomiting (1,2). Currently, corticosteroids are used adjuvant to certain chemotherapy regimens, either as an antiemetic, to reduce other side effects, or for additional therapeutic benefit (3). Corticosteroids are also frequently dispensed to patients with end stage disease who may no longer be eligible for anti-cancer therapies as a method of symptom control (3). However, corticosteroids, such as dexamethasone, prednisone, and hydrocortisone, have been known to cause elevations in blood glucose levels in both patients with and without diabetes (4-6).
Hyperglycemia may lead to acute complications or adverse events (AEs), such as dehydration, increased risk of infection, ketoacidosis, and acute hyperglycaemic syndrome (7). It has also been shown to be a risk factor for cancer-related death in men, and was found to have a significant negative effect on survival in patients with small cell lung and breast carcinomas (7,8). As such, blood glucose levels may need to be monitored in patients both with and without diabetes receiving corticosteroids along with their chemotherapy regimen.
In order to gain an understanding of the frequency of blood glucose and/or glycosylated hemoglobin (HbA1c) monitoring of patients receiving concurrent corticosteroids with their chemotherapy regimen, a retrospective analysis was undertaken.
Methods
Patients with genitourinary (GU) cancers treated at the Sunnybrook Odette Cancer Centre in Toronto, Canada from July to December 2013 with at least one chemotherapy cycle inclusive of a corticosteroid used both for premedication and continuously throughout the treatment period were eligible for the study. Patient medical records were retrospectively reviewed to extract patient demographics, chemotherapy treatment details, corticosteroid treatment dates and dosages, and diabetes status prior to start of treatment. All laboratory blood glucose tests [either fasting plasma glucose (FPG), or random plasma glucose (RPG)], or HbA1c, were extracted from on- and accessible off-site records and were analyzed applying the Canadian Diabetes Association (CDA) criteria for diabetes mellitus (DM) diagnosis and pre-diabetes diagnosis (9). RPG and FPG results were organized into the four graded categories set forth by the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Home blood glucose tests were not included due to the retrospective nature of this study. Glucose monitoring was defined as receiving a blood test before the first chemotherapy administration as well as a test within a week of each subsequent treatment cycle. At the time of this review, there were no guidelines for glucose monitoring in patients receiving corticosteroids with chemotherapy. All tests were ordered at the discretion of the patients’ oncologist. We also undertook a literature review (Table 1). This study was approved by the Research Ethics Board prior to commencement.

Full table
Results
Patient demographics
A total of 30 GU male patients were eligible, of which 37% (n=11) had diabetes and 63% (n=19) did not have diabetes prior to receiving corticosteroid treatment. Of the 30 GU patients, 26 were prostate cancer (PC) patients, and four were bladder cancer patients. The median body mass index (BMI) was 29.5 kg/m2 for patients with diabetes and 26.5 kg/m2 for those without diabetes. The median ages were 74 and 69 years, respectively. All patients received docetaxel chemotherapy with oral prednisone 5 mg (continuous) and dexamethasone 8 mg the day prior to and the day of chemotherapy treatment. The median incidence of blood glucose monitoring was 13% and 75% in patients with and without diabetes, respectively. The incidence of hyperglycemia among patients in each group is summarized in Table 2.
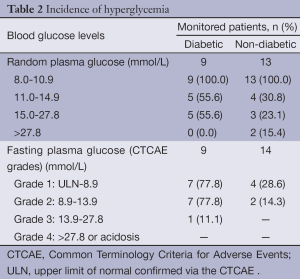
Full table
Patients without pre-existing diabetes
Hyperglycemia resulted in hospitalization in 23% (n=7) of monitored patients. Thirteen patients without diabetes were tested with RPG, of which 15% (n=3) had a RPG greater than 27.8 mmol/L. Two of these patients were admitted to hospital, and then subsequently discharged with diabetic medications. The other patient presented with polyuria and polydipsia and was admitted to hospital with hyperosmolar hyperglycemia due to the addition of dexamethasone to their medication regime. The patient was started on insulin, educated on home glucose monitoring and discharged. Later, the patient was readmitted to hospital with elevated blood glucose levels and cholangitis; the patient subsequently declined rapidly and expired.
A further 15% (n=3) of patients without diabetes had a RPG result in the range of 15.0-27.8 mmol/L. Two of these patients were admitted to hospital; one was admitted with acute multiple organ failure, possibly due to tumor lysis syndrome, as well as with concurrent severe metabolic acidosis. He expired in hospital. The other patient was admitted for hyperglycemia and discharged with a reduced dexamethasone dose to improve glycemic control. One patient without diabetes required a change in prednisone dosing to achieve better glycemic control.
Patients with pre-existing diabetes
For patients with diabetes with a RPG result above 11.0 mmol/L, 40% (n=4) required a change in their diabetes management and 20% (n=2) required hospitalizations where hyperglycemia was noted as a comorbidity impacting hospital stay. One patient admitted for sepsis and poor urinary control was noted to have inadequate glycemic control resulting in an endocrinology consult and adjustment of insulin dosage. The other patient was admitted for acute kidney injury due to urinary retention and was discharged home without modification of his diabetes regimen. One week post-corticosteroid commencement an additional individual was seen in the emergency department with a FPG result of 26.7 mmol/L. Insulin was added to his diabetes regimen along with home blood glucose monitoring.
Discussion
The effects of corticosteroids are observed within hours of administration and are generally dose dependent, impacting RPG levels more so than FPG levels (19). High doses and prolonged use of corticosteroids may result in side effects such as hyperglycemia, increased insulin resistance, dyslipidemia, osteoporosis, adrenal suppression, cardiovascular disease (CVD), Cushing’s syndrome, psychological disturbances, and immunosuppression (19-21). Acute hyperglycemia is known to impair immune function and wound healing, which represents a risk to cancer patients by lengthening hospital stays and increasing risk of all-cause mortality (14,21). The results of this study identified potential areas of improvement for the management of corticosteroid-associated hyperglycemia. The findings of the study may help in guiding the formation of screening guidelines to be implemented at the centre in order to optimize patient care. To prevent complications and potential AEs in cancer patients receiving corticosteroid therapy best practice guidelines should include parameters for glycemic control.
A study by Yoo et al. (4) examined 632 patients who received docetaxel chemotherapy and were screened for hyperglycemia (defined as 2 RPG levels >11.1 mmol/L) and for infectious events requiring antibiotics. Two separate blood glucose tests with levels above 11.1 mmol/L will conclude the patient has diabetes, not merely hyperglycemia (9). The study determined the incidence of hyperglycemia in all patients was 13.8% and risk factors for hyperglycemia were being overweight or obese, and previous DM. On the other hand, the study determined corticosteroid dose intensity and density, as well as the chemotherapy administration schedule were not risk factors for hyperglycemia, making it difficult to identify high risk patients based on treatment regimen. An observational cohort study conducted by Harris et al. screened 90 patients undergoing chemotherapy plus concurrent corticosteroid treatment for brain tumors or metastases, for lymphomas, or as part of bone marrow transplants (14). Patients were monitored with RPG tests and hyperglycemia was found in 59% of all patients and 19% tested in the diabetic range. The investigators found no correlation between risk factors for diabetes and the individuals who experienced hyperglycemia, thus recommending all cancer patients were screened 4-6 hours post steroid administration. Thus it may be difficult to determine high risk patients for developing corticosteroid-induced hyperglycemia (C-IH) and hard to prevent if typical risk factors for diabetes are not consistent in this circumstance. It may be surmised that if the individual tests are normal after initial dosing, then they may not require additional monitoring. The aforementioned cancer population tended to receive higher doses of corticosteroids than the GU cancer patients in our study. This may explain the higher incidence of hyperglycemia. Thus, any screening recommendations may need to be modified for GU cancer patients.
The Joint British Diabetes Association (JBDA) recently published guidelines for inpatient care, which state that predisposing factors for steroid induced hyperglycemia include pre-existing type 1 or 2 diabetes, obesity, family history of diabetes, as well as others (22). It is essential that risk factors for corticosteroid-induced diabetes are identified so that high risk patients, who may require more rigorous monitoring, can be determined. The risk factors proposed by Yoo et al. (4) and the JBDA may be applicable in this approach.
Brady et al. (5) conducted a literature review on C-IH and determined that hyperglycemia occurs in hospitalized cancer patients irrespective of diabetic history. The study concluded that all corticosteroid recipients should be monitored through clinical observation and blood glucose tests where appropriate. Two other studies previously determined that C-IH is common in both patients with and without diabetes [odds ratio of 1.5 to 2.5 and 1.36 to 2.31 for developing glucocorticoid-induced hyperglycemia (GC-IH) in patients treated with a glucocorticoid (GC), respectively] and determined that total GC dose and duration of use were strong predictors of GC induced diabetes, along with age and BMI (6,23). Furthermore, even acute elevations in blood glucose levels may be associated with adverse outcomes (6). Both studies concluded that steroids can result in significant clinical implications that require early recognition and management in patients both with and without diabetes (6).
A recent study by Derweesh et al. (24) recommended blood glucose screening 48 hours after GC initiation followed by screening every 3-6 months for the first year then annually thereafter. This may represent a more feasible model for monitoring patients on long term low dose corticosteroid use, such as PC patients undergoing chemotherapy or androgen synthesis inhibitor therapy with abiraterone. However, due to the high incidence of hyperglycemia and hospitalizations observed in this cohort, more rigorous and frequent testing may be required for chronic high dose corticosteroids. Liu et al. (19) recommended all patients have extensive baseline examination including medical history, body weight, height, and blood pressure to determine risk factors or conditions that may be exacerbated by corticosteroid use. A blood glucose test should be ordered before initiating corticosteroid therapy, if initial results are abnormal at baseline then home glucose monitoring is recommended (19). The CDA recommends that any individual commencing corticosteroid therapy be monitored for the subsequent 48 hours and glycemic control maintained, regardless of pre-existing diabetes or not (9). If individuals test above the recommended target range (6-10 mmol/L) then interventions may be required and individuals should continue to be screened. In particular, males with metastatic castrate resistant PC treated with corticosteroids should be closely monitored for the presence of steroid associated toxicities, including hyperglycemia, with the possibility of adjusting the dose in certain affected patients (25).
Brunello et al. (16) reviewed 349 non-Hodgkin lymphoma (NHL) (n=162) and PC (n=187) patients treated with a chemotherapy regimen inclusive of a steroid. Abnormal glucose levels at baseline were found in 44% and 68% of NHL and PC patients, respectively. Over the course of treatment, dysglycemia was observed in 70% of patients with NHL and 92% of patients with PC. The study found no association between hyperglycemia and survival nor hospitalization rate (16). A similar result was determined by Hong et al. (11) who reviewed 206 metastatic colorectal patients segregated into four graded categories according to mean blood glucose results (using all available tests). Increasing mean blood glucose was associated with more infection related AEs, but not shorter survival. Various other studies have suggested that pre-existing diabetes in addition to cancer increases all-cause mortality and cancer recurrence in various cancer types (7,8,14,26,27). In a recent meta-analysis, Barone et al. (26) determined that patients with pre-existing diabetes with endometrial, breast, or colorectal cancer had a 41% increased risk of cancer mortality compared to individuals without diabetes. Therefore, it is essential that proper diabetes management is part of the standard of care for cancer patients with diabetes or pre-diabetes to ensure best standard of practice and optimal patient outcomes. The studies reporting on C-IH have discrepant results regarding effect on survival, yet tend to be unanimous that hyperglycemia can lead to AEs. Future studies should be conducted to determine the extent of the corticosteroid adverse effects on patient survival.
Patients with pre-existing diabetes who receive corticosteroids as part of their cancer treatment may require a change in their diabetes management and consideration of the benefits and risks before commencement of steroid therapy. Duan et al. (28) hypothesized that hyperglycemia contributes to malignant growth by inhibiting apoptosis, inducing cell proliferation, aiding metastasis, and facilitating chemotherapy resistance. Individuals receiving corticosteroids require some form of monitoring to prevent acute AEs, as well as long term complications in non-palliative patients. Patients who develop corticosteroid-induced diabetes may require endocrinologist assessment and commencement of anti-hyperglycemics.
In our study, a cohort of 30 cancer patients treated with a chemotherapy regimen inclusive of a corticosteroid were retrospectively evaluated for frequency of blood glucose monitoring and prevalence of hyperglycemic events. Our study may underestimate the clinical significance and incidence of abnormal blood glucose levels as not all patients were monitored consistently. However, due to the retrospective nature of our study we were not able to determine the incidence of home capillary blood glucose monitoring, and if any individual treatment or monitoring modifications occurred. It is also unknown if patients were symptomatic when blood testing occurred, or if any intervention was conducted by the physician ordering the glucose test. The Joint British Diabetes Societies recently came out with recommended monitoring guidelines for steroid-induced diabetes, as well as recommendations for glycemic control in hospitalized patients and outpatients. The guidelines recommend for patients without diabetes that monitoring occurs once per day with the frequency of testing related to the glucose level measured. For patients with diabetes, testing should occur 4 times per day (22). If both patient groups exceed 12 mmol/L on two separate occasions then treatment should be dispensed (22). The guideline provides a well-structured monitoring regime, yet will require extensive patient education and resources to supply all patients who take steroids with home capillary glucose monitoring kits. Additionally, a study conducted by Zanders et al. showed that adherence to glucose-lowering drug treatment declines following a cancer diagnosis (29). The introduction of more medications and treatment regiments may result in overall lower adherence to drug treatment. The CTCAE uses FPG for the grading of hyperglycemic events, which we applied to RPG tests in this study. It must also be noted that FPG test results may be erroneous due to confounding factors, such as patient non-compliance to fasting.
Conclusions
Corticosteroids are an essential component of the current standard of care for treatment of certain types of cancer. The chronic use of corticosteroids may exacerbate existing diabetic status and worsen treatment outcomes for cancer patients. Patients who are prescribed a corticosteroid should be monitored in order to prevent adverse effects and to be able to intervene if they occur. Future studies should ascertain the optimal method of monitoring in patients undergoing chronic corticosteroid use; one that is feasible and is able to prevent corticosteroid- induced AEs. Investigations should also determine the threshold for clinical significance and the effect on cancer outcomes from abnormal blood glucose levels. To optimize patient care and outcomes patients receiving corticosteroids should receive adequate support and monitoring in order to prevent the development of corticosteroid-induced diabetes and its associated complications.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Disclosure: The authors declare no conflict of interest.
References
- Dick GS, Meller ST, Pinkerton CR. Randomised comparison of ondansetron and metoclopramide plus dexamethasone for chemotherapy induced emesis. Arch Dis Child 1995;73:243-5. [PubMed]
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008;358:2482-94. [PubMed]
- Auchus RJ, Yu MK, Nguyen S, et al. Use of prednisone with abiraterone acetate in metastatic castration-resistant prostate cancer. Oncologist 2014;19:1231-40. [PubMed]
- Yoo KE, Kang RY, Lee JY, et al. Awareness of the adverse effects associated with prophylactic corticosteroid use during docetaxel therapy. Support Care Cancer 2014. [Epub ahead of print]. [PubMed]
- Brady VJ, Grimes D, Armstrong T, et al. Management of steroid-induced hyperglycemia in hospitalized patients with cancer: a review. Oncol Nurs Forum 2014;41:E355-65. [PubMed]
- Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract 2009;15:469-74. [PubMed]
- Weiser MA, Cabanillas ME, Konopleva M, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer 2004;100:1179-85. [PubMed]
- Luo J, Chen YJ, Chang LJ. Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients. Lung Cancer 2012;76:242-7. [PubMed]
- Canadian Diabetes Association Guidelines. Canadian Journal of Diabetes. Available online: http://guidelines.diabetes.ca/App_Themes/CDACPG/resources/cpg_2013_full_en.pdf
- Lee SY, Kurita N, Yokoyama Y, Seki M, Hasegawa Y, Okoshi Y, Chiba S. Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer 2014;22:1385-90. [PubMed]
- Hong YJ, Han HS, Jeong Y, et al. Impact of hyperglycemia on survival and infection-related adverse events in patients with metastatic colorectal cancer who were receiving palliative chemotherapy. Cancer Res Treat 2014;46:288-96. [PubMed]
- Kotila TR, Olutogun T, Ipadeola A. Steroid induced diabetes mellitus in patients receiving prednisolone for haematological disorders. Afr Health Sci 2013;13:842-4. [PubMed]
- Jensen K, Steinthorsdottir KJ, Brandt B. In-hospital cardiac arrest due to unobserved steroid-induced hyperglycaemic hyperosmolar syndrome. Ugeskr Laeger 2013;175:1044-5. [PubMed]
- Harris D, Barts A, Connors J, et al. Glucocorticoid-induced hyperglycemia is prevalent and unpredictable for patients undergoing cancer therapy: an observational cohort study. Curr Oncol 2013;20:e532-8. [PubMed]
- Gonzalez-Gonzalez JG, Mireles-Zavala LG, Rodriguez-Gutierrez R, et al. Hyperglycemia related to high-dose glucocorticoid use in noncritically ill patients. Diabetol Metab Syndr 2013;5:18. [PubMed]
- Brunello A, Kapoor R, Extermann M. Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. Am J Clin Oncol 2011;34:292-6. [PubMed]
- Gannon C, Dando N. Dose-sensitive steroid-induced hyperglycaemia. Palliat Med 2010;24:737-9. [PubMed]
- Krone CA, Ely JT. Controlling hyperglycemia as an adjunct to cancer therapy. Integr Cancer Ther 2005;4:25-31. [PubMed]
- Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol 2013;9:30. [PubMed]
- Dupuy DE, Liu D, Hartfeil D, et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer 2010;116:989-97. [PubMed]
- Cheng Z, Wang K. Influence of chemotherapy on blood glucose in cancer patients. Chinese J Clin Oncol 2009;36:1194-6.
- Joint Britich Diabetes Societies for inpatient care. Management of Hyperglycemia and Steroid (Glucocorticoide) Therapy. Available online: http://www.diabetologists-abcd.org.uk/JBDS/JBDS_IP_Steroids.pdf
- Kwon S, Hermayer KL. Glucocorticoid-induced hyperglycemia. Am J Med Sci 2013;345:274-7. [PubMed]
- Derweesh IH, Diblasio CJ, Kincade MC, et al. Risk of new-onset diabetes mellitus and worsening glycaemic variables for established diabetes in men undergoing androgen-deprivation therapy for prostate cancer. BJU Int 2007;100:1060-5. [PubMed]
- Dorff TB, Crawford ED. Management and challenges of corticosteroid therapy in men with metastatic castrate-resistant prostate cancer. Ann Oncol 2013;24:31-8. [PubMed]
- Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 2008;300:2754-64. [PubMed]
- Paschou SA, Leslie RD. Personalizing guidelines for diabetes management: twilight or dawn of the expert? BMC Med 2013;11:161. [PubMed]
- Duan W, Shen X, Lei J, et al. Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int 2014;2014:461917.
- Zanders MM, Haak HR, van Herk-Sukel MP, et al. Impact of cancer on adherence to glucose-lowering drug treatment in individuals with diabetes. Diabetologia 2015;58:951-60. [PubMed]


