
The influence of STK11 mutation on acquired resistance to immunotherapy in advanced non-small cell lung cancer with Lynch syndrome: a case report and literature review
Introduction
Over the past decade, immune checkpoint inhibitors (ICIs) have changed the treatment paradigm for non-small cell lung cancer (NSCLC). Some characteristics have been investigated as positive predictive biomarkers for ICI therapy, including programmed death ligand 1 (PD-L1), microsatellite instability-high (MSI-H), tumor mutational burden (TMB), and human leukocyte antigen (HLA). Moreover, a segment of patients is unable to respond well to programmed cell death protein (PD-1)/PD-L1 inhibitors. Previous research has suggested that EGFR mutations, ALK fusion, STK11 mutations, and loss of PTEN are related to poor response (1-4). However, the existence of acquired resistance mechanisms remains unclear. Although colorectal cancer with Lynch syndrome might respond well to ICIs (5), the effectiveness of ICIs in lung cancer is uncertain due to the distant relationship between lung cancer and Lynch syndrome (6). In this paper, we report a patient diagnosed with advanced lung squamous cell carcinoma with a background of Lynch syndrome and high PD-L1 expression. Pembrolizumab monotherapy was administered as a first-line treatment and successfully reduced the tumor size. However, the disease progressed, and an STK11 frameshift mutation was found in the patient’s plasma circulating tumor deoxyribonucleic acid (ctDNA) 6 months later. This case raises a few important questions that deserve further investigation: (I) could STK11 contribute to immunotherapy’s acquired resistance mechanism? (II) Does Lynch syndrome play a role in this case? We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1639).
Case presentation
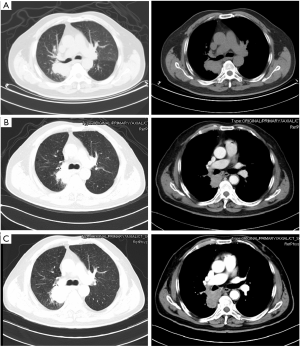
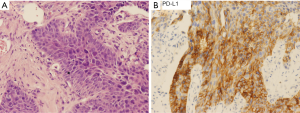
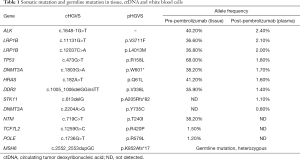
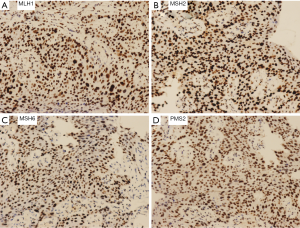
A 76-year-old Chinese male presented with a cough and bloody sputum in November 2017. He had a 50-year smoking history and a family history of gastric cancer (brother) and colon cancer (sister). He had no previous history of other disease. The image results during diagnosis and immunotherapy were shown in Figure 1. Computed tomography (CT) of the chest showed masses in the upper lobe and hilar of right lung, accompanied by mediastinal lymphadenopathy (Figure 1A). There were no other signs of distant metastasis observed by positron emission tomography–CT (PET-CT). Bilateral bronchial abnormalities were found by fibrobronchoscopy, and squamous cell carcinomas were identified by the lower left and upper right lung biopsies (Figure 2A). PD-L1 was highly expressed (50%) in bilateral lung biopsies, which were elucidated by immunohistochemistry (IHC) using an anti-PD-L1 antibody (22C3) (Figure 2B). Next-generation sequencing (NGS) using 1,021 cancer-related genes (Appendix 1) was performed on the tissue. Ten mutations including HRAS c.182A>T (p.Q61L) (allele fraction/AF, 41.2%) and TP53 c.473G>T (p.R158L) (AF, 68.0%) were detected (Table 1), and STK11 was identified as wild-type. There was no targetable variation, and the tumor mutational burden (TMB) was 8.0Muts/Mb, which was rated as low (from the Geneplus database). Unexpectedly, one heterozygous germline mutation, MSH6 c.2552_2553dupGC (p.K852Afs*17), was detected in the white blood cells (Table 1, Figure 3). However, the IHC results suggested normal expression of MLH1, MSH2, MSH6 and PMS2 in his tumor biopsy, and it was classified as proficient mismatch repair (pMMR) (Figure 4). Based on the hereditary aspect of Lynch syndrome, genetic counseling for his daughter and granddaughter found an equivalent mutation sequence in MSH6.
Full table
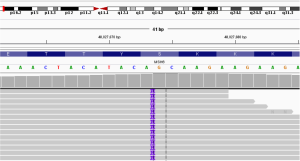
The patient was diagnosed with stage IV right lung squamous cell carcinoma (cT3N3M1a). Pembrolizumab (200 mg, ivgtt, q3w), a monoclonal anti-programmed cell death protein 1 (PD-1) antibody, was commenced in November 2017. After four pembrolizumab cycles, chest CT showed that the right hilar mass had decreased by approximately 22%, and the lymph nodes were unchanged (Figure 1B). The Response Evaluation Criteria in Solid Tumours (RECIST, v1.1) efficacy evaluation indicated stable disease (SD). However, disease progression (PD) occurred shortly after eight pembrolizumab cycles had been administered (Figure 1C). The plasma ctDNA test at PD revealed nine somatic mutations, and an STK11 c.613delG (p.A205Rfs*82) (AF, 1.1%) frameshift mutation was newly identified compared with the primary tissue (Table 1, Figure 5). Common terminology criteria for adverse events (CTCAE) assessment revealed grade 1 hepatic injury due to pembrolizumab treatment. The toxicity and side effects were tolerable. Subsequently, the patient received chemotherapy with albumin-bound paclitaxel.

This study was approved by the ethics committee of West China Hospital of Sichuan University (No.587, 2018). All procedures performed in this study involving human participants were following the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
ICI monotherapy or combination therapies have demonstrated survival advantage in patients with advanced NSCLC (2,7,8). Predictive biomarkers for immunotherapy are still under exploring. Retrospective analysis has suggested that high PD-L1 expression and MSI-H were associated with a higher objective response rate (ORR) to PD-1/PD-L1 inhibitors (9), however ALK rearrangement and EGFR mutation usually does not benefit from immunotherapy (10). Loss of PTEN, JAK1/2, and amplification of MDM2/4 are been reportedly associated with anti-PD-1/PD-L1 resistance or hyperprogressors in other tumors (11-13).
STK11 is an onco-suppressor gene that is frequently inactivated in 8% of NSCLCs (14). Koyama et al. demonstrated reduced expression of PD-L1 in a genetically engineered murine model (GEMM) of KRAS-driven/STK11-deficient NSCLC compared to KRAS-driven/STK11-normal status, and STK11-deficient tumors were found to be resistant to the anti-PD-1 antibody (15). STK11-inactivating mutations can modulate the immune milieu of the lung tumor microenvironment, and offer specific implications for addressing STK11-mutated tumors with PD-1-targeting antibody therapies (16). Targeted NGS results from 240 NSCLC patients who were treated with a PD-1/PD-L1 antibody suggested that STK11 variants were significantly enriched in the no durable benefit (NDB) group compared to the non-ICI NSCLC group (17). The studies above suggest primary (early) resistance to immunotherapy in patient with an STK11 mutation. This raises a question regarding whether the STK11 mutation might be one of the acquired (late) resistance mechanism of immunotherapy. Truncating mutations of B2M and JAK1/2 have been distinguished as acquired resistance factors of PD-1 blockade immunotherapy in melanoma (13). However, in this case, STK11 was a newly identified mutation in the patient’s plasma, which was not found in the primary tissue. Thus, the relationship between STK11 mutation and acquired resistance to pembrolizumab in NSCLC requires further research.
Hereditary nonpolyposis colorectal cancer (HNPCC), also called Lynch syndrome, is an autosomal dominant familial cancer syndrome caused by MMR gene germline mutation. MMR gene “second-hit” has been frequently found in Lynch syndrome-related cancers, and mostly with MSI-H (18). Furthermore, colorectal cancer with MSI-H has shown a superior response to immunotherapy (5). Although an MSH6 germline mutation was identified in this NSCLC patient, the MMR proteins were normally expressed. However, whether there is any correlation between Lynch syndrome and lung cancer remains unclear as the data regarding Lynch syndrome and lung cancer is limited. The patient had a family history of gastric and colon cancers, which are related to Lynch syndrome. Considering that Lynch syndrome increases the risk of developing various cancers, the patient should monitor for a second tumor, and family members who have MSH6 germline mutation should pay attention to cancer screenings.
Our study describes a high PD-L1 expression NSCLC patient with an MSH6 germline mutation, who was treated with pembrolizumab as first-line therapy. The patient’s disease progressed in 6 months. An STK11 frameshift mutation was newly-discovered in his plasma by NGS. Moreover, the coincidental discovery of Lynch syndrome added complexity to clinical diagnosis and treatment. The impact of STK11, Lynch syndrome, and PD-L1 expression on the efficiency of PD-1 inhibitors requires further discussion and research.
Conclusions
At present, there are too many unknowns in cancer immunotherapy. This case provided one possibility that STK11 frameshift mutations may contribute to pembrolizumab secondary resistance. Dynamic gene surveillance in plasma might contribute to efficacy prediction during cancer immunotherapy. Given its scarcity, lung cancer with Lynch syndrome is rarely reported, however, the correlation between them requires further research.
Acknowledgments
We owe thanks to the patient and his family.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1639
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1639). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of West China Hospital of Sichuan University (No.587, 2018). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chowell D, Morris LGT, Grigg CM, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2018;359:582-7. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Hellmann MD, Callahan MK, Awad MM, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2019;35:329. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Bogner B. Predictive markers of immunotherapy of colorectal cancer. Magy Onkol 2019;63:192-5. [PubMed]
- Shukuya T, Patel S, Shane-Carson K, et al. Lung Cancer Patients with Germline Mutations Detected by Next-Generation Sequencing and/or Liquid Biopsy. J Thorac Oncol 2018;13:e17-9. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Lu S, Stein JE, Rimm DL, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol 2019;5:1195-204. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res 2017;23:4242-50. [Crossref] [PubMed]
- George S, Miao D, Demetri GD, et al. Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity 2017;46:197-204. [Crossref] [PubMed]
- Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375:819-29. [Crossref] [PubMed]
- Facchinetti F, Bluthgen MV, Tergemina-Clain G, et al. LKB1/STK11 mutations in non-small cell lung cancer patients: Descriptive analysis and prognostic value. Lung Cancer 2017;112:62-8. [Crossref] [PubMed]
- Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov 2015;5:860-77. [Crossref] [PubMed]
- Koyama S, Akbay EA, Li YY, et al. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res 2016;76:999-1008. [Crossref] [PubMed]
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol 2018;36:633-41. [Crossref] [PubMed]
- Peiró G, Diebold J, Lohse P, et al. Microsatellite instability, loss of heterozygosity, and loss of hMLH1 and hMSH2 protein expression in endometrial carcinoma. Hum Pathol 2002;33:347-54. [Crossref] [PubMed]
(English Language Editors: A. Kassem and J. Chapnick)


