
Sinonasal manifestations and dynamic profile of RT-PCR results for SARS-CoV-2 in COVID-19 patients
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first described in December 2019 in Wuhan, China (1-3). At present, the disease has rapidly spread to more than 200 counties across the world (4). Several studies have reported that COVID-19 can not only infect the lower respiratory tract with symptoms of fever, dry cough and dyspnea but also cause multiple systemic infections (5-10). In the literature, the rate of sinonasal symptoms among COVID-19 patients is highly inconsistent. Initial studies of COVID-19 reported a low prevalence of sinonasal symptoms, ranging from 4–8% (1,4,11). In contrast, other studies from Australia and America reported much higher prevalence of sinonasal symptoms among COVID-19 patients (12,13). Moreover, it has become apparent that some sinonasal symptoms appear early and may be considered a potential predictor of COVID-19 (14).
However, to our knowledge, the clinical manifestation of sinonasal symptoms in COVID-19 remains incompletely characterized. The aim of the present study was to characterize the prevalence, timing, and severity of sinonasal symptoms, as well as the dynamic profile of real-time reverse transcription polymerase chain reaction (RT-PCR) results for SARS-CoV-2, in a longitudinal fashion.
We present the following article in accordance with the MDAR checklist and the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2493).
Methods
Patient population
This was a retrospective, observational study in which 11 consecutive patients with COVID-19 were recruited from January 22 to March 18, 2020 at Third Hospital of Baotou City (Inner Mongolia, China). All enrolled patients were confirmed to be diagnosed with COVID-19 according to China’s “pneumonia diagnosis and treatment program of novel coronavirus infection (trial version 6)” (15). The next follow-up date was April 1,2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of Beijing Tongren Hospital and Third Hospital of Baotou City (No.: 2020-0413 and 2020-002, respectively) and informed consent was taken from all individual participants.
Data sources
The clinical symptoms and RT-PCR results for SARS-CoV-2 were extracted from electronic medical records. The diagnosis of a history of chronic rhinosinusitis (CRS) and allergic rhinitis (AR) were based on the current recommendations (16,17). Data on sinonasal symptoms included the start and end times of each symptom and worst symptom severity during COVID-19. Individual patient-assessed symptoms were assessed at their most severe level by a visual analog scale (VAS), which ranged from 0 (not troublesome) to 10 (worst thinkable), with total scores of 0 to 3 indicating the presence of mild symptoms, 3 to 7 indicating moderate symptoms, and 7 to 10 indicating severe symptoms (18). The diagnosis of sinonasal manifestations mainly depended on the subjective symptoms of the patients and the available examinations. Any missing or uncertain records were collected and clarified with the involved patients over the phone, on an online form or in the patient’s room. According to China's “pneumonia diagnosis and treatment program of novel coronavirus infection (trial version 6)” (15), the severity of COVID-19 was divided into mild, moderate, severe and critical at its worst during the disease course. Mild disease of COVID-19 referred to mild clinical symptoms and no signs of pneumonia on imaging and moderate state referred to having fever, respiratory symptoms, and imaging showing pneumonia. For adults, severe state must meet any of the following: (I) shortness of breath occurred, respiratory rate was greater than or equal to 30 times/min; (II) at rest, the oxygen saturation was less than or equal to 93%; (III) arterial partial pressure of oxygen/inhaled oxygen concentration was less than or equal to 300 millimeters of mercury. Critical state of COVID-19 must meet any of the following: (I) respiratory failure occurred and required mechanical ventilation; (II) shock occurred; (III) combined with other organ failure, intensive care unit monitoring and treatment was required. The RT-PCR results of SARS-CoV-2 were collected and analyzed from the patients' nasal and oropharyngeal swabs, sputum, and urine and stool specimens over time. The primers and probes of RT-PCR detection kit for specific detection of SARS-CoV-2 were selected from regions of Open Reading Frame 1ab (ORF1ab) and nucleocapsid (NP) gene of SARS-CoV-2 genome according to the manufacturer’s protocol (Jiangsu Shuoshi Biotechnology Co., Ltd.). When two targets (ORF1ab, NP) tested positive by specific RT-PCR, the case would be considered to be laboratory confirmed. A cycle threshold value (Ct-value) less than 37 was defined as a positive test, and a Ct-value of 40 or more was defined as a negative test. A medium load, defined as a Ct-value of 37 to 40, required confirmation by retesting.
Statistical analysis
The SPSS statistical software (version 22.0) was used for all statistical analyses (SPSS, Inc., Chicago, IL, USA). Continuous variables are directly expressed as the mean, median and interquartile range (IQR) values. Comparisons of numerical variables between groups were conducted using the Mann-Whitney U test. Categorical variables are presented as frequencies (%) and were compared using the χ2 test or Fisher’s exact test between groups. All tests were two-sided, and P<0.05 was considered statistically significant.
Results
Demographic and Epidemiologic characteristics of COVID-19 patients
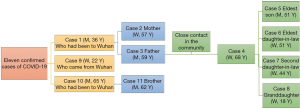
A total of 11 hospitalized patients with confirmed SARS-CoV-2 infections were included in the analysis. Their demographic and clinical characteristics are shown in Table 1. Two family clusters are shown in Figure 1.
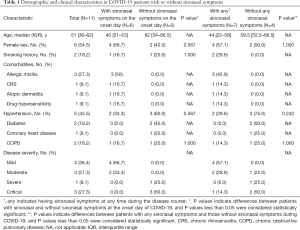
Full table
Prevalence, timing and severity of sinonasal symptoms in COVID-19 patients
At baseline, two patients who had accompanied with seasonal AR had no sinonasal symptoms. One patient who had a combined history of AR and CRS, had a stable mild self-reported sinonasal symptoms at baseline but the severity of sinonasal symptoms was aggravated during the period of COVID-19. The other patients had no sinonasal symptoms at baseline. In addition, none of them had a history of previous sinonasal surgeries prior to the onset of COVID-19.
The spectrum of the main symptoms is shown in Figure 2. Seven (63.6%) patients presented with sinonasal manifestations, and the most common sinonasal symptoms during the entire disease course were nasal obstruction [7 (63.6%)], rhinorrhea and olfactory dysfunction [5 (45.5%)]. Six patients (54.5%) presented sinonasal manifestations on the day of onset, and nasal obstruction [6 (54.5%)] was the most common symptom, followed by rhinorrhea [5 (45.5%)] and olfactory dysfunction [4 (36.4%)]. All six patients with sinonasal symptoms on the day of onset had non-severe infections, with mild infections in 66.7% of these patients and moderate infections in 33.3%. The majority of patients with any sinonasal symptoms mainly had non-severe infections, with mild infection in 57.1% of these patients, moderate infection in 28.6% and critical infection in 14.3%. The prevalence of isolated anosmia amongst the study population was 9.09%.
The timing of main symptoms of these patients is shown in Figure 3. Sinonasal symptoms including nasal obstruction, rhinorrhea and olfactory dysfunction, as well as cough and fever commonly appeared early, while other symptoms, such as phlegm, short of breath and sore throat, often appeared late. The median time from the onset of illness to nasal obstruction or olfactory dysfunction was 0 (IQR, 0–4) days. The patients with rhinorrhea presented this symptom on the day of onset. The four-week recovery rates from sinonasal symptoms were 85.7% for nasal obstruction, 80% for rhinorrhea and 66.7% for olfactory dysfunction. We further found that case #3, who had a history of AR and CRS, had a longer recovery time (over 40 days) from nasal obstruction, rhinorrhea and olfactory dysfunction. Notably, case #8, who experienced olfactory and subsequent gustatory dysfunction during COVID-19, still has not recovered to the pre-disease level and developed a distorted taste of certain flavors.
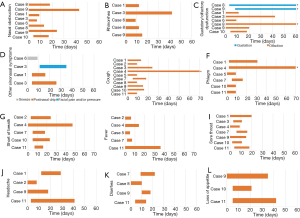
Figure 4 shows the worst reported severity of sinonasal symptoms during the course of COVID-19. All patients with nasal obstruction reported moderate severity, and 80% reported moderate rhinorrhea and a moderately decreased sense of smell. Twenty percent of patients who experienced rhinorrhea reported mild rhinorrhea, while 20% of patients who experienced olfactory dysfunction reported severe olfactory dysfunction.
Dynamic profile of SARS-CoV-2 detected by RT-PCR over the course of COVID-19
The total number of SARS-CoV-2 RT-PCR results for the 11 patients was 365 (average of 33 tests per patient). The RT-PCR results of urine samples were all negative during the course of COVID-19. The RT-PCR results of the various biological samples from the 11 patients over a seven-week period, starting from the onset of symptoms, are presented in Table 2. Because the patients without any sinonasal symptoms were transferred to the intensive care unit in another hospital for further treatment, some RT-PCR results for SARS-CoV-2 were not available. The overall rate of positive RT-PCR results from all specimens during the entire period was highest in the first week [11/15 (73.3%)] and then gradually decreased over time. However, 3 patients (27.3%) had experienced a long-lasting fluctuated positive RT-PCR results since 29 d.a.o in both groups, especially for the two patients with airway comorbidities (case #4 had a comorbidity of COPD and case #3 had a combined history of AR, CRS and dermatitis).
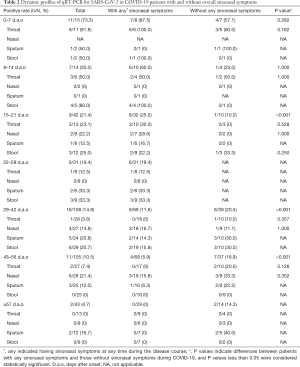
Full table
Compared to patients without sinonasal symptoms, patients with sinonasal symptoms presented a higher rate of positive RT-PCR results from all specimens collected 15–21 d.a.o (25% vs. 10%, P<0.001). However, the rate of positive RT-PCR results from all specimens collected 29–56 d.a.o. was lower in patients with sinonasal symptoms than in patients without sinonasal symptoms.
The current guidelines suggest that two consecutive negative RT-PCR test results are a criterion for discharge. According to the local policy, all patients meeting the discharge criteria were discharged and then transferred to another isolation ward in the same hospital for centralized isolation until two consecutive RT-PCR tests were negative every 2 weeks after discharge. Regarding the fluctuating positive RT-PCR results from sputum samples, case #4 was still in the hospital for further management as of our data collection deadline.
Discussion
According to the local anti-epidemic policy in Inner Mongolia, all patients should receive necessary follow-up in a centralized isolation department. Therefore, we had sufficient time to understand the dynamic changes of these patients in a longitudinal manner. To the best of our knowledge, this is the first report to detail the sinonasal manifestations and dynamic RT-PCR changes of SARS-CoV-2 in patients with COVID-19.
In our study, we found that sinonasal symptoms attributed to COVID-19 were more than 50%, which is in contrast to the findings of a previous study conducted in China (1,5,11). However, the reported prevalence of sinonasal symptoms was higher in Australia, USA and Europe, ranging from 30–50% (11,13,19,20). We analyzed the different prevalences of sinonasal symptoms, which may be partly attributed to the different disease statuses of COVID-19 patients, different levels of attention paid to nonspecific sinonasal complaints by patients and physicians, the diversity of angiotensin-converting enzyme 2 (ACE2) expression patterns, mutations of the virus, the differences in affinity of some viruses to certain tissues and individuals and environmental or seasonal factors (19,21,22). Another finding was that patients with sinonasal symptoms were more likely to have mild or moderate disease than patients without sinonasal symptoms. Sinonasal symptoms of COVID-19 may be related to disease severity. However, this hypothesis needs further investigation. Of note, case #4 in our study presented with isolated sinonasal symptoms, without any other symptoms during the course of COVID-19, suggesting that some individuals could be hidden carriers of SARS-CoV-2, as they do not meet the current criteria for a diagnosis of COVID-19; this may support the role of otolaryngologists as first-line physicians for some COVID-19 patients.
We then explored the timing profile of sinonasal symptoms attributed to COVID-19. We found that the majority of sinonasal symptoms appeared early, which was consistent with a previous study (14). In addition, patients had a relatively poor recovery rate from olfactory and gustatory dysfunction. Hence, sinonasal symptoms may appear early and should be cared for by physicians and patients, making the symptoms important for the early detection of this disease, and some sinonasal symptoms may be long lasting, causing symptoms that require aggressive treatment.
We also analyzed the dynamic profile of RT-PCR results for SARS-CoV-2 in various biological specimens over the course of COVID-19. Our study confirmed the findings of prior studies that the rate of positive RT-PCR results in all specimens is highest in the first week (23). Patients with sinonasal symptoms seemed to have a relatively different rate of positive RT-PCR results for SARS-CoV-2 in all specimens over the course of testing, but this needs further study. Only 2 patients (18.2%) showed at least one positive result for SARS-CoV-2 in nasal specimens, and this rate seemed unrelated to the occurrence of sinonasal symptoms. The positive rate of patients with sinonasal symptoms who exhibiting at least one positive result for nasal specimens was lower than that of patients without sinonasal symptoms (14.3% vs. 25%). Additionally, patients with underlying airway comorbidities seemingly presented with prolonged corresponding symptoms and had long-lasting positive RT-PCR results for SARS-CoV-2. However, these hypotheses still need further investigation in a larger population. The “re-positive” nucleic acid tests after discharge were attributed to intermittent virus excretion, an insufficient drug treatment regimen and false-negative RT-PCR test results due to differences in the sources of the collected samples, the method of sample collection, antiviral drugs or hormones administered, sample transportation, test methods and sensitivity of nucleic acid test kits (24-26). Although traces of SARS-CoV-2 detected by RT-PCR were not necessarily correlated with the ability of transmission, a longer observation period should be considered for certain groups of COVID-19 patients (25,27,28).
The results of our study should be interpreted within the constraints of its limitations. This study is limited by the small sample size and retrospective method. We also acknowledge that our study design relied heavily on adequate patient recall and reports. However, previous studies of recall bias suggest that the recall of disease-specific symptoms, in particular those related to noteworthy events (such as COVID-19), is generally reliable, particularly for short periods. In addition, we should improve the accuracy of the detection protocol, master the sampling method and regularly collect various biological specimens within the same period. Further research is required to investigate the relationship of RT-PCR results and symptom onset and overall symptoms on a large scale. Therefore, a prospective study with strict inclusion criteria and more clinicopathological measurements on a large scale are needed to validate these findings.
In conclusion, since COVID-19 is new and the pathogenesis is much complicated, as of yet, there remain more questions than answers. In our study, sinonasal symptoms were more prevalent in patients with mild or moderate COVID-19 and usually appeared at the beginning of the disease course. The rate of patients who presented positive RT-PCR results for SARS-CoV-2 in nasal specimens seemed unrelated to the incidence of sinonasal symptoms. Regular nucleic acid testing for SARS-CoV-2 should be considered for COVID-19 patients with certain airway comorbidities.
Acknowledgments
Funding: This study was supported by the National Key R&D Program of China (No. 2016YFC0905200); the Program for the Changjiang Scholars and Innovative Research Team (No. IRT13082); the National Natural Science Foundation of China (No. 81630023, 81970852); the Beijing Bai-Qian-Wan talent project (No. 2019A32); the Public Welfare Development and Reform Pilot Project (No. 2019-10); and the CAMS Innovation Fund for Medical Sciences (No. 2019-I2M-5-022).
Footnote
Reporting Checklist: The authors have completed the MDAR checklist and the STROBE checklist. Available at http://dx.doi.org/10.21037/apm-20-2493
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2493
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2493). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of Beijing Tongren Hospital and Third Hospital of Baotou City (No.: 2020-0413 and 2020-002, respectively) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382:727-33. [Crossref] [PubMed]
- Zu ZY, Jiang MD, Xu PP, et al. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology 2020;296:E15-E25. [Crossref] [PubMed]
- Zhu J, Zhong Z, Ji P, et al. Clinicopathological characteristics of 8697 patients with COVID-19 in China: a meta-analysis. Fam Med Community Health 2020;8:e000406 [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res 2020;7:11. [Crossref] [PubMed]
- Kowalski LP, Sanabria A, Ridge JA, et al. COVID-19 pandemic: Effects and evidence-based recommendations for otolaryngology and head and neck surgery practice. Head Neck 2020;42:1259-67. [Crossref] [PubMed]
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417-8. [Crossref] [PubMed]
- Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683-90. [Crossref] [PubMed]
- Chen L, Liu M, Zhang Z, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol 2020;104:748-51. [Crossref] [PubMed]
- Chang D, Lin M, Wei L, et al. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA 2020;323:1092-3. [Crossref] [PubMed]
- COVID-19 National Incident Room Surveillance Team. COVID-19, Australia: Epidemiology Report 7 (Reporting week ending 19:00 AEDT 14 March 2020). Commun Dis Intell (2018) 2020; [Crossref]
- Speth MM, Singer-Cornelius T, Oberle M, et al. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol Head Neck Surg 2020;163:114-20. [Crossref] [PubMed]
- Krajewska J, Krajewski W, Zub K, et al. COVID-19 in otolaryngologist practice: a review of current knowledge. Eur Arch Otorhinolaryngol 2020;277:1885-97. [Crossref] [PubMed]
- Pneumonia diagnosis and treatment program of novel coronavirus infection (trial version 6). National health commision of the people’s republic of China; 2020. Available online: http://www.nhc.gov.cn/yzygj/s7653p/202002/ 8334a8326dd94d32 9df35 1d7da8aefc2.shtml. Accessed March 7, 2020.
- Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020;58:1-464. [Crossref] [PubMed]
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008;63:8-160. [Crossref] [PubMed]
- Bachert C, Mannent L, Naclerio RM, et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients With Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA 2016;315:469-79. [Crossref] [PubMed]
- Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020;277:2251-61. [Crossref] [PubMed]
- Mercante G, Ferreli F, De Virgilio A, et al. Prevalence of Taste and Smell Dysfunction in Coronavirus Disease 2019. JAMA Otolaryngol Head Neck Surg 2020;146:1-6. [Crossref] [PubMed]
- Benvenuto D, Giovanetti M, Ciccozzi A, et al. The 2019-new coronavirus epidemic: Evidence for virus evolution. J Med Virol 2020;92:455-9. [Crossref] [PubMed]
- Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov 2020;6:11. [Crossref]
- Xiao AT, Tong YX, Gao C, et al. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: A descriptive study. J Clin Virol 2020;127:104346 [Crossref] [PubMed]
- Cao H, Ruan L, Liu J, et al. The clinical characteristic of eight patients of COVID-19 with positive RT-PCR test after discharge. J Med Virol 2020;92:2159-64. [Crossref] [PubMed]
- Li Y, Yao L, Li J, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol 2020;92:903-8. [Crossref] [PubMed]
- Liu R, Han H, Liu F, et al. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta 2020;505:172-5. [Crossref] [PubMed]
- Xiao AT, Tong YX, Zhang S. False-negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: Rather than recurrence. J Med Virol 2020;92:1755-6. [Crossref] [PubMed]
- Lan L, Xu D, Ye G, et al. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA 2020;323:1502-3. [Crossref] [PubMed]




