
Refusal of medical treatment by older adults with cancer: a systematic review
Introduction
Cancer is a global health problem of great magnitude, particularly in older patients. A considerable number of older adults are diagnosed with this disease. For older adults, an increase of 67% in cancer incidence is anticipated, compared with younger adults (11% increase). Also, 60% of the mortality cases due to cancer are estimated to occur in people aged 70 and older (1-3).
To date, the perspective of older patients with cancer regarding oncology treatment is an unexplored field. A review about decision-making on cancer treatment showed that research is still poor regarding treatment decision in older adults (4). Refusal therapy and underuse of treatment are more common in older adults, and they are associated with worst outcomes such as higher rates of cancer recurrence and worst survival, even knowing that cancer-directed treatments have evolved and that more “elderly-friendly” treatments have been developed (3). Furthermore, the heterogeneity of this population’s health and functional status may interfere in treatment preferences and must be taken into account (3-5).
Many studies have explored the actual role in the decision-making process; however, most were conducted years ago and with a relatively young population. With advances in cancer treatment and more available options for the elderly population, older adults may have different views than those included in previous studies (5).
One of the principles of good care is respect and receptivity to patients’ wishes and values; thus, it is important to understand patients’ perspectives about cancer treatment. Most studies have focused on the decision-making processes related to the choice between cancer-directed therapies and palliative care (5,6). However, little is known about the factors associated with treatment refusal in oncology. Some preliminary data suggest that therapeutic refusal is partially based on balancing the pros and cons of treatment (6).
Thus, the aim of this review was to identify the factors associated with treatment refusal by older adults with cancer. We expect that this systematic review provides knowledge regarding the clinical and sociodemographic characteristics associated with therapeutic refusal in this population.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2439).
Methods
Search strategy
We conducted a systematic review starting with the search in three databases in July 2020, namely Medline (PubMed), Web of Knowledge (ISI) and Scopus, using the key concepts: “refusal treatment” and “cancer treatment” and “decision making” and “elderly” or “aged”.
Inclusion criteria
Studies that included the factors or reasons for refusing cancer therapy in the elderly (65 years or older), not necessarily exclusively to the elderly as long as there are reported results on a subgroup analysis of older adults. We consider ‘therapeutic refusal’ any cancer treatment, such as surgery, chemotherapy, radiotherapy, as well as curative or palliative treatment.
We included studies written in English, French, Portuguese and Spanish languages. Empirical articles, quantitative and qualitative studies were also included. Given that a similar review was conducted in 2015, we considered studies from 2015 until July 2020 for updated scientific and research developments in this area.
Exclusion criteria
Editorials, letters to the editor, reviews, comments and narrative case reports were excluded. Studies on other related topics, such as transition of care and no indication of active treatment for advanced disease, were also excluded. We considered only those studied related to a patient’s refusal of therapy.
Quality assessment of studies and data extraction
Study quality and eligibility were individually assessed by the two researchers (LD, MRB). The extraction of data was done manually without extraction software. There was a critical review of the results by the researchers (LD, MRB) and coordinators (WFB, FR). In case of different opinions regarding articles’ relevance, a consensus was reached by the authors. Data systematization was evaluated by the periodical (title, volume, number and year), study location country, objectives (article or search), method (type of research, sample, participants), results, and mention of treatment refusal. Final assessment of the quality and level of evidence and the recommendation force of the articles, when absent in the original articles, were conducted by the authors, according to the criteria of the Strength of Recommendation Taxonomy scale by the American Family Physician (7) (Table S1).
Results
Through the search strategy, 221 studies were initially identified: 67 in PubMed, 58 in ISI, and 87 in Scopus. Ten studies were found in other databases because they were found in the articles’ references and met the eligibility criteria for this study.
The PRISMA (Preferred Reporting Items For Systematic Reviews and Meta-Analyses) flow diagram ame (8) is shown in Figure 1. Screening covered the stages of analysis by titles and summary. Based on the title and abstract, 139 articles (screening) were excluded. In addition, 45 duplicate articles were excluded. A total of 37 full-text articles were analyzed, of which 14 were excluded as they did not respect the study criteria. A total of 22 articles met the inclusion criteria and were included in this review (Table 1).
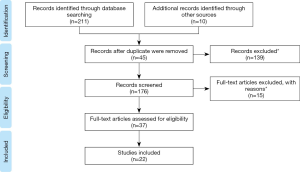
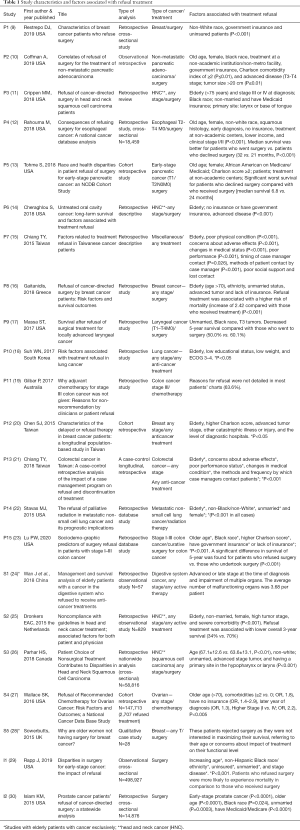
Full table
Characteristics of the studies and quality assessment
The included studies’ characteristics are shown in Table 1 and information about the quality assessment is described in Table S1. Nineteen studies used a retrospective, cross-sectional analytical design, and at least thirteen had controlled data and were compared with a case group (patients who refused treatment) and case control (patients who received treatment). Thirteen studies (9-14,16,20,22,23,26,27,30) were non-randomized but with a large sample size, with data collected from National Database, thus representing a nationwide population. Almost all of the studies used retrospective chart reviews and/or an administrative database. There was just one study with a qualitative design (28), with a sample size of 28 patients. The sample size for quantitative studies ranged from 58 to 498,927 patients (24,29). Study quality ranged from poor to moderate for most studies (level 2 and 3 evidence; Appendix A. The majority of studies focused on head and neck (11,14,17,25,26), breast (9,16,20,28), and colorectal cancers (19,21,23). There were two studies on lung cancer (18,22) and two on pancreatic cancer (10,13). Three studies included different types of cancer (miscellaneous) (15,24,29). Most of the studies were from the United States (9-14,17,22,23,27,29,30). There were five Asian (15,18,20,21,24) and three European studies (16,25,28) There were no studies from Latin America.
Predictive factors associated with refusal treatment in the elderly
Socio-demographic factors
Only two studies included elderly patients exclusively (24,28): one included only elderly women with breast cancer and the other one included elderly people with digestive cancer. The vast majority of studies (10-16,18,20,22,23,26,27,29) showed that being elderly was an important refusal factor. The factors associated with treatment refusal were mostly unmarried status (11,16,17,22,25-27,29,30) female gender (10,12-14,22,25), non-white race (9-13,16,17,23,26,29,30) having government insurance (9-11,13,14,23,30), or not having insurance (9,14,16,23,27,29).
Types of intervention
The majority of studies evaluated refusal of surgery interventions (9-14,16,17,23,26,28-30). Two studies evaluated refusal of chemotherapy (19,27). One article included palliative treatment (radiotherapy) exclusively, and showed that older patients with non-small cell lung cancer (NSCLC) with metastases were more likely to refuse radiation therapy (22).
Clinical characteristics
Most studies showed that clinical status was a predictive factor associated with treatment refusal. Cancer stage III or IV (10,11,14,16,17,24-27) was more associated with refusal treatment, as well as poor performance status (Eastern Cooperative Oncology Group 3 or 4) and Charlson comorbidity score >2 (10,13,15,18,20,21,23-25,27).
Discussion
Although there have been few studies on the treatment refusal of the older patient with cancer, the majority of studies analyzed described old age as one of the strongest predictive factors associated with therapy refusal. A national cohort study of lung cancer in Taiwan described that the rate of treatment refusal increased proportionally for each 10-year age increase, and among patients aged 75 and older was increased 2.6 times compared to those aged 44 or under (18,31). Administration of chemotherapy decreased with age and increasing number of comorbidities. Walter et al. (32) showed in a sample of patients with stage III colon cancer in Germany that old age was the strongest predictor of non-administration of chemotherapy, irrespective of comorbidity and other potential determinants. Evidence suggests that the increase in patient refusal rate corroborates with the still apparent issue of undertreatment in healthy older patients (3).
The factors associated with treatment refusal found in this review were unmarried status (11,16,17,25-27,29,30) non-white race (9-13,16,17,23,26,29,30) female gender (10,12-14,22,25), having government insurance (9-11,13,14,23,30) or having no insurance (9,14,16,23,27,29) and Charlson comorbidity index >2 (10,13,15,18,20,21,23,24,27). These results are aligned with the factors shown recently by Rapp et al. (29), although this British study only included patients who were recommended for surgery for early-stage disease (primary stage I and II lung, breast, prostate and colon cancers). However, the reasons behind these clinical-demographic factors were not well established in these studies, as the vast majority were descriptive studies (9-14,16,17,22,23,27,29,30).
Regarding the demographic factors, Chiang et al. (21), Suh et al. (18), and Chiang et al. (15) also showed that patients older than 70 years old, widowed or unemployed were more likely to refuse treatment. Poor social support and lower income were also described as factors related with treatment refusal (12,13,28). Furthermore, non-white race and female gender appeared to be strong predictive factors of therapy refusal (9,10,12-14,22,25,28). Despite the absence of a clear explanation in literature, we speculate that regional and cultural characteristics may influence the decision-making treatment. A relationship between race, age and unmarried status and patient refusal of surgery for lung cancer was found in a US study, suggesting cultural differences in decision-making regarding treatment (33). The patient’s cultural background and socioeconomic status can impact the desire to overcome cancer in a particular country or region. Low educational and economic status was considered a significant variable in treatment refusal, maybe due to difficulty in accessing health services or lack of knowledge about disease (18). Patients perceive and experience illness, care, and death according to their culture, values, beliefs, life experiences and meaning of life (34). Thus, it is argued that spirituality, culture, the socioeconomic status and policies of access to healthcare may influence patients’ healthcare decision-making (35). In this review, the five Asian studies (15,18,20,21,24) did not described the female gender as a predictive factor, which was mostly described by American studies. We did not find studies that included Latin American elderly patients.
The clinical factors associated with refusal treatment reported in the majority of the studies were mainly advanced disease stage (III or IV) (10,11,14,16,17,24-27) Charlson score greater than two (10,13,15,18,20,21,23-25,27), and poor performance status (ECOG 3 or 4) (15,18,21,23,25). This may be related to the desire not to undergo treatments without a curative proposal considering advanced disease (22,25). Only one systematic review was found about factors associated with acceptance and refusal of cancer treatment in the elderly. The results showed that the physician’s recommendation, trusting and having good communication with the physician, and expectations about side effects were predictive factors of therapy refusal, with the latter representing the main factor related to treatment refusal that was also found in the current review (3,21,28,29).
The majority of studies in this review did not examine the particular reasons for older adults to decline treatment (9,10,12-14,16-18,20,21,25,27). Thus, it is unclear if other factors were not reported because they were not important or because they were not been studied. Relevant characteristics of geriatric health, such as cognitive and sensory impairment, were not described in these articles, but were found in the previous systematic review (3). So, it is important that multicenter studies with a larger sample size and of higher methodological quality are conducted, considering the clinical characteristics and aspects of patients, such as their culture, values, beliefs. As well as qualitative studies to better understand the reasons for therapeutic refusal (3,24).
A 2015 qualitative study in the United Kingdom (28), including only elderly women with breast cancer who refused surgery, reported that these patients declined surgery either because they wanted to avoid treatments that could impact their current functional level or because of their age perception. These patients, who tended to be older, mentioned their age as a representation of various reasons, including having a limited life span, not wishing to prolong their life due to comorbidities or lack of desire. In this review, there is only one qualitative study that evaluated the psychological and existential aspects of treatment refusal. Juang et al. suggested that one of the reasons for treatment refusal is that older patients may have lack of knowledge about their medical conditions; thus, they may feel uncertain about the disease, which can lead to depression and affect patients’ adherence to treatment (36).
Compared to the latest systematic review on the topic (3), we found some differences. In that review, most studies used a qualitative design based on breast and prostate cancer treatment decisions, and described as important factors associated with therapeutic refusal the concerns with the discomfort of the treatments, fear of side effects and transportation barriers. In this study, most studies used a retrospective and descriptive design and focused on breast, colorectal, and head and neck cancers. The differences related to the predictive factors between the two reviews could be influenced by the nature of the studies’ design. The qualitative and prospective design prevalent in the first review (3) favors the discussion of psychological aspects such as fear of adverse effects.
It is important to consider that not all studies in this review involved only curative treatment. One study had a palliative proposal for non-small cell lung cancer, which also showed some factors of refusal described in this review such as elderly, unmarried, and female patients. Stavas (22) and Aizer et al. (37) compared refusal characteristics of patients undergoing curative and palliative care and they found that older and unmarried patients were more likely to decline palliative and curative radiation, demonstrating that these are stable predictors of refusal, independent of treatment indication. Regarding gender, women were more likely to refuse palliative radiotherapy (RT) and men were more prone to refuse definitive RT (22). Unfortunately, the database used in most studies in this review [the Surveillance Epidemiology and End Results (SEER) and National Cancer Database] (9-14,16,17,22,23,27,29-31) did not give further information to detail these differences. Thus, further investigation into the demographic factors related with treatment refusal should be conducted.
Regarding the type of treatment (10-18,20,21,23-26,28-30), the majority of studies evaluated the refusal of surgery interventions, which may be associated with the nature of the cancer. Most of the studies included head and neck, breast, and digestive system early-stage cancer treatment, where surgery has a well-established role in treatment (10-14,16,17,26,28-30). Seven studies (12,13,16,17,23,25,29) described an overall lower survival rate of patients who refused treatment compared with those who received standard treatment, most of them regarding surgery intervention. Lu et al. (23) showed that old age was a predictive factor of refusing surgery in patients with stage I-III colon cancer. The previous study has also described that elderly patients were more likely to decline surgery for other neoplasms when compared to younger patients, even though they were suitable surgical candidates. About the reasons for declining surgery, Rothman et al. (38) described that most older patients with advanced illness refused a surgical/medical procedure and the main reason was the fear of side effects.
It is know that the integration of palliative care improves several outcomes, such as quality of life and symptom burden and less use of medical resources (39). The American Society of Clinical Oncology has recommended that palliative care should be offered since cancer diagnosis, together with traditional oncologic care. Although the benefits of early palliative care are already well established scientifically, there are challenges to its implementation, such as the current health care policies, limited resources, and the different clinical practice settings, which consequently lead to palliative care referrals occuring late in the illness process (40). Although all patients with metastatic disease or poor performance status would benefit from palliative care, identifying those individuals who refuse treatment is also a good indication for referral (22,39).
Further research is needed to understand why these disparities exist for treatment refusal, especially in patients with early-stage cancer. Most studies are retrospective, which makes it difficult to analyze the reasons behind treatment decision-making. Some possible explanations include the lack of healthcare literacy and mistrust of individual medical providers and of the overall healthcare system described by minority patients. The two articles (24,28) that exclusively enrolled older patients showed that in particular, patients 80 years and older may be more likely to refuse surgical intervention due to fear of decreased quality of life, as they may be affected by other conditions that lead to frailty, while the unmarried patients might be more likely to decline treatment because of a perception of a poor social support, which also was observed in prior studies (29,41).
This review has some limitations. The methodological quality of the included studies limited some of the findings. The retrospective and descriptive nature of most of the studies made it difficult to clarify the reasons behind treatment refusal. No meta-analysis was conducted, as the studies were too heterogeneous regarding the population studied and data collected. The strengths of this study were the nature of the systematic review that included both quantitative and qualitative studies, most of which were from large nationwide and multicentric databases (9-14,16,20,22,23,26,30,31) and up-to-date about an issue that is still scarce in the literature.
Conclusions
This study reviewed the factors associated with treatment refusal in older patients with cancer. Predictive factors of refusal treatment included female gender, unmarried status, non-white race, having government insurance or not having insurance, higher disease stage, and poor performance status (9-18,20-27,30). Understanding these factors is important in clinical practice to improve treatment adherence, reduce errors, improve results and provide optimal care, considering the best interests and values of patients (3,6,10). Thus, acknowledging patients’ specific demographic and clinical characteristics may help to predict patients’ attitudes towards decision-making in health and allow to elaborate an adequate care plan, also considering patients’ culture and personal values and beliefs. Additional studies are needed with elderly patients to evaluate decision-making, particularly regarding the psychological and existential aspects, and incorporating both health literacy and comorbidity.
Acknowledgments
We would like to express our sincere appreciation to the authors who performed all eligible studies included in the present study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2439
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-2439
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2439).The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758-65. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2017
- Puts MT, Tapscott B, Fitch M, et al. A systematic review of factors influencing older adults' decision to accept or decline cancer treatment. Cancer Treat Rev 2015;41:197-215. [Crossref] [PubMed]
- Santoni G, Angleman S, Welmer AK, et al. Age-related variation in health status after age 60. PLoS One 2015;10:e0120077 [Crossref] [PubMed]
- Sattar S, Alibhai SMH, Fitch M, et al. Chemotherapy and radiation treatment decision-making experiences of older adults with cancer: A qualitative study. J Geriatr Oncol 2018;9:47-52. [Crossref] [PubMed]
- Huijer M, Van Leeuwen E. Personal values and cancer treatment refusal. J Med Ethics 2000;26:358-62. [Crossref] [PubMed]
- Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-56. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 [Crossref] [PubMed]
- Restrepo DJ, Sisti A, Boczar D, et al. Characteristics of Breast Cancer Patients Who Refuse Surgery. Anticancer Res 2019;39:4941-5. [Crossref] [PubMed]
- Coffman A, Torgeson A, Lloyd S, et al. Correlates of Refusal of Surgery in the Treatment of Non-metastatic Pancreatic Adenocarcinoma. Ann Surg Oncol 2019;26:98-108. [Crossref] [PubMed]
- Crippen MM, Elias ML, Weisberger JS, et al. Refusal of Cancer-Directed Surgery in Head and Neck Squamous Cell Carcinoma Patients. Laryngoscope 2019;129:1368-73. [Crossref] [PubMed]
- Rahouma M, Harrison S, Kamel M, et al. Consequences of Refusing Surgery for Esophageal Cancer: A National Cancer Database Analysis. Ann Thorac Surg 2018;106:1476-83. [Crossref] [PubMed]
- Tohme S, Kaltenmeier C, Bou Samra P, et al. Race and Health Disparities in Patient Refusal of Surgery for Early-Stage Pancreatic Cancer: An NCDB Cohort Study. Ann Surg Oncol 2018;25:3427-35. [Crossref] [PubMed]
- Cheraghlou S, Kuo P, Mehra S, et al. Untreated oral cavity cancer: Long-term survival and factors associated with treatment refusal. Laryngoscope 2018;128:664-9. [Crossref] [PubMed]
- Chiang TY, Wang CH, Lin YF, et al. Factors Related to Treatment Refusal in Taiwanese Cancer Patients. Asian Pac J Cancer Prev 2015;16:3153-7. [Crossref] [PubMed]
- Gaitanidis A, Alevizakos M, Tsalikidis C, et al. Refusal of Cancer-Directed Surgery by Breast Cancer Patients: Risk Factors and Survival Outcomes. Clin Breast Cancer 2018;18:e469-76. [Crossref] [PubMed]
- Massa ST, Osazuwa-Peters N, Franco J, et al. Survival after refusal of surgical treatment for locally advanced laryngeal cancer. Oral Oncol 2017;71:34-40. [Crossref] [PubMed]
- Suh WN, Kong KA, Han Y, et al. Risk factors associated with treatment refusal in lung cancer. Thorac Cancer 2017;8:443-50. [Crossref] [PubMed]
- Gilbar P, Lee A, Pokharel K, et al. Why adjuvant chemotherapy for stage III colon cancer was not given: Reasons for non-recommendation by clinicians or patient refusal. J Oncol Pharm Pract 2017;23:128-34. [Crossref] [PubMed]
- Chen SJ, Kung PT, Huang KH, et al. Characteristics of the delayed or refusal therapy in breast cancer patients: A longitudinal population-based study in Taiwan. PLoS One 2015;10:e0131305 [Crossref] [PubMed]
- Chiang TY, Wang CH, Lin YF, et al. Colorectal cancer in Taiwan: A case-control retrospective analysis of the impact of a case management programme on refusal and discontinuation of treatment. J Adv Nurs 2018;74:395-406. [Crossref] [PubMed]
- Stavas MJ, Arneson KO, Ning MS, et al. The refusal of palliative radiation in metastatic non-small cell lung cancer and its prognostic implications. J Pain Symptom Manage 2015;49:1081-7.e4. [Crossref] [PubMed]
- Lu PW, Fields AC, Yoo J, et al. Sociodemographic predictors of surgery refusal in patients with stage I‐III colon cancer. J Surg Oncol 2020;121:1306-13. [Crossref] [PubMed]
- Wan J, Xu S, Wu Y, et al. Management and survival analysis of elderly patients with a cancer in the digestive system who refused to receive anticancer treatments. Support Care Cancer 2018;26:2333-9. [Crossref] [PubMed]
- Dronkers EA, Mes SW, Wieringa MH, et al. Noncompliance to guidelines in head and neck cancer treatment; associated factors for both patient and physician. BMC Cancer 2015;15:515. [Crossref] [PubMed]
- Parhar HS, Anderson DW, Janjua AS, et al. Patient Choice of Nonsurgical Treatment Contributes to Disparities in Head and Neck Squamous Cell Carcinoma. Otolaryngol Head Neck Surg 2018;158:1057-64. [Crossref] [PubMed]
- Wallace SK, Lin JF, Cliby WA. Refusal of Recommended Chemotherapy for Ovarian Cancer: Risk Factors and Outcomes; a National Cancer Data Base Study. J Natl Compr Canc Netw 2016;14:539-50. [Crossref] [PubMed]
- Sowerbutts AM, Griffiths J, Todd C, et al. Why are older women not having surgery for breast cancer? A qualitative study. Psychooncology 2015;24:1036-42. [Crossref] [PubMed]
- Rapp J, Tuminello S, Alpert N, et al. Disparities in surgery for early-stage cancer: the impact of refusal. Cancer Causes Control 2019;30:1389-97. [Crossref] [PubMed]
- Islam KM, Wen J. Prostate cancer patients' refusal of cancer-directed surgery: a statewide analysis. Prostate Cancer 2015;2015:829439 [Crossref] [PubMed]
- Huang HL, Kung PT, Chiu CF, et al. Factors associated with lung cancer patients refusing treatment and their survival: A national cohort study under a universal health insurance in Taiwan. PLoS One 2014;9:e101731 [Crossref] [PubMed]
- Walter V, Boakye D, Weberpals J. Decreasing Use of Chemotherapy in Older Patients With Stage III Colon Cancer Irrespective of Comorbidities. J Natl Compr Canc Netw 2019;17:1089-99. [Crossref] [PubMed]
- Mehta RS, Lenzner D, Argiris A. Race and health disparities in patient refusal of surgery for early-stage non-small cell lung cancer: A SEER cohort study. Ann Surg Oncol 2012;19:722-7. [Crossref] [PubMed]
- Rego F, Rego G, Nunes R. Moral agency and spirituality in palliative care. Ann Palliat Med 2020;9:2286-93. [Crossref] [PubMed]
- Rego F, Pereira C, Rego G, et al. The Psychological and Spiritual Dimensions of Palliative Care: A Descriptive Systematic Review. Neuropsychiatry (London) 2018;8:484-49. [Crossref]
- Juang YY, Lin CR, Yang TY, et al. Depressive disorders in patients with cancer. Formosan J Med 2013;17:155-62.
- Aizer AA, Chen MH, Parekh A, et al. Refusal of curative radiation therapy and surgery among patients with cancer. Int J Radiat Oncol Biol Phys 2014;89:756-64. [Crossref] [PubMed]
- Rothman MD, Van Ness PH, O'Leary JR, et al. Refusal of medical and surgical interventions by older persons with advanced chronic disease. J Gen Intern Med 2007;22:982-7. [Crossref] [PubMed]
- Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302:741-9. [Crossref] [PubMed]
- Yoong J, Park ER, Greer JA, et al. Early palliative care in advanced lung cancer: a qualitative study. JAMA Intern Med 2013;173:283-90. [Crossref] [PubMed]
- Gopal N, Kozikowski A, Barginear MF, et al. Reasons for chemotherapy refusal or acceptance in older adults with cancer. South Med J 2017;110:47-53. [Crossref] [PubMed]


