
Use of dexamethasone and a 5-HT3 receptor antagonist with or without aprepitant to prevent chemotherapy-induced nausea and vomiting among patients with lung cancer who are treated with platinum-based chemotherapy: a systematic review and meta-analysis of randomized controlled trials
Introduction
Lung cancer (LC) is the most widespread cancer in the world and has higher mortality rates than all other cancers (1,2). Guidelines for treating LC (3,4) indicate that the first-line treatment for LC is platinum-based chemotherapy (PBC). This treatment has been confirmed to be beneficial for patients. However, chemotherapy-induced nausea and vomiting (CINV) is the most common adverse event (5). CINV exerts a significant negative effect on quality of life (QoL) and even stops patients from undergoing chemotherapy treatment (6). Dexamethasone and one type of 5-HT3 receptor antagonist (DH) is commonly used as a standard and classical antiemetic treatment to prevent CINV. However, some of its effects have yet to be confirmed in the clinic.
Aprepitant is a neurokinin-1 (NK-1) RA that functions in both the gut and the central nervous system by antagonizing the interaction of substance P (chemotherapy-related increase) and NK-1 receptors to prevent CINV (7). Some clinical trials have suggested that adding aprepitant (ADH) to DH has achieved excellent effects (8). However, researchers have not clearly determined whether ADH is better than DH at preventing CINV in patients with LC (9,10). Several trials have been conducted to examine this issue. In one randomized controlled trial (RCT), Wu reported a better overall complete response (CR) and a lower rate of rescue antiemetic treatment (RAT) in the ADH group, but the no nausea rate (NNR) was similar in the two groups (11). Aksu also reported that ADH led to a better CR and lower Functional Living Index Emesis (FLIE) scores in patients with LC (12). However, Kusagaya and Ito reported similar CRs and NNRs in the acute, delayed and overall phases between the ADH and DH groups (13,14).
A meta-analysis was performed to compare the CR and NNR of patients with LC treated with PBC to examine the effects and safety of ADH and DH.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2290).
Methods
This meta-analysis was performed in accordance with the PRISMA guidelines (15,16) (Registration information: PROSPERO CRD42020162227).
Search strategy
The following eight databases were used to search for relevant articles: (I) PubMed, (II) ScienceDirect, (III) The Cochrane Library, (IV) Scopus, (V) Web of Science, (VI) Embase, (VII) Ovid MEDLINE, and (VIII) Google Scholar.
Two researchers conducted separate searches of the databases and manual searches in duplicate to search for all relevant research documents published between January 1990 and July 2020. Our search was conducted from database inception to July 30, 2020. “Aprepitant”, “Dexamethasone”, and “Chemotherapy” were used as key terms (Figure S1) illustrates the search strategy. The reference lists of the retrieved articles were manually searched for additional relevant studies. All searches were performed without language barriers.
Selection criteria
We formulated the inclusion criteria in accordance with the principle of PICOS: (I) population: patients who have been diagnosed with LC and treated with PBC were guided by the Eastern Cooperative Oncology Group (ECOG); (II) intervention and comparison: ADH versus DH; (III) outcomes: CR, NNR, RAT, adverse events (AEs) and FLIE score; (IV) study design: RCTs. We excluded reviews without raw data (conducted by screening the full text), meta-analyses, animal-based experiments, abstracts, and duplicate studies.
Data extraction
The following data were collected and summarized separately by two researchers (MYH and RXX): primary author’s name, publication date, research period, nation, study planning, number of recipients, recipient characteristics (age, sex, ECOG status, treatment arm, chemotherapy regimen, and pathological type), efficacy indices (CR, NNR, RAT, and FLIE score) and AEs. A third reviewer resolved all the discrepancies (ZWX).
Outcome assessments
The CR and NNR were assessed as the primary outcomes. The CR was subdivided into the overall CR (days 1 to 5), acute CR (day 1) and delayed CR (days 2 to 5). The NNR was subdivided into the overall NNR, acute NNR and delayed NNR.
We performed a further subgroup analysis stratified according to the severity and type of AEs. For severity, AEs were classified into total AEs and grade 3–5 AEs. AE types were divided into hematological toxicity (leukopenia, neutropenia, anemia, and thrombocytopenia) and nonhematological toxicity (hepatotoxicity, nephrotoxicity, constipation, allergic reaction, hiccups, fatigue, diarrhea, decreased appetite, and abdominal pain).
Quality assessment
The Cochrane Risk of Bias Tool (CRBT) and Jadad scale were applied to assess the quality of RCTs. The evaluation indicators of the CRBT include randomization sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting. Study quality was classified as high, low, or unclear (17). Three key terms are included in the evaluation indicators of the Jadad scale: randomness, blackout, and follow-up management. A score ≥3 indicates high quality (18).
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was used to examine the quality of the outcomes. The evaluation indicators include the risk of bias, nonuniformity, inconsistency, inaccuracy and publication bias. The outcome quality was classified as high, medium, low or very low (19).
Statistical analysis
Review Manager (Ver. 5.4) and STATA (Ver. 15.1) software were used to carry out the meta-analysis. P≤0.05 was considered statistically significant. Data were summarized using risk ratios (RRs) with 95% CIs for the CR, NNR, FLIE scores (RR >1 prefers ADH; RR <1 prefers DH), and the number of patients who needed RAT (RR >1 prefers DH; RR <1 prefers ADH). We considered P<0.05 a statistically significant difference. Mantel-Haenszel (MH) was used for pooling variances (17). An AE analysis was performed to indicate the distinction among each reported symptom. Subgroup analyses of the CR and NNR were performed to identify in which scenario the outcomes varied based on the nation, chemotherapy regimen, 5-HT3 RA type, ADC (%) and performance status. We used Begg’s and Egger’s tests to explore publication bias. Heterogeneity was assessed using the χ2 test and I2 statistic. Significant heterogeneity was indicated by the results of the χ2 test as being significant at P<0.1 or by an I2>50%, and in these cases, the random effects model was adopted; otherwise, the fixed effects model was adopted.
Results
Search results and study quality assessment
We preliminarily screened 2118 potential qualified studies. Four studies assessing 518 patients (ADH group, 261 patients; DH group, 257 patients) were ultimately included in the analysis (Figure 1) (11-14). All the studies were RCTs. One study was conducted in China, two were conducted in Japan, and one was conducted in Turkey. The study characteristics are presented in Table 1. Based on the CRBT and Jadad scores, all of the included trials were of high quality (Figure S2 and Table S1). According to the GRADE framework, all the outcomes were of high or medium quality (Table S2).
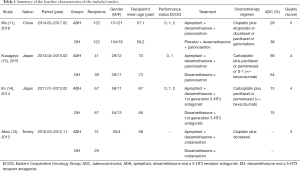
Full table
Complete response
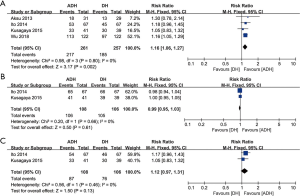
We considered nonvomiting and nonuse of emergency medicines as CR standards and assessed the efficacy of the CR based on the overall CR, acute CR and delayed CR. Four studies compared the overall CR (heterogeneity: P=0.80, I2=0%). The ADH group had a significantly better CR than the DH group (RR: 1.16, 95% CI: 1.06–1.27, P=0.002; Figure 2A). Two studies compared the acute CR (heterogeneity: P=0.66, I2=0%). No significant difference was observed between groups (RR: 0.99, 95% CI: 0.95–1.03, P=0.61; Figure 2B). Two studies compared the delayed CR (heterogeneity: P=0.46, I2=0%), and the ADH group showed a better CR, but the difference was not significant (RR: 1.12, 95% CI: 0.97–1.31, P=0.13, I2=0%; Figure 2C).
No nausea rate
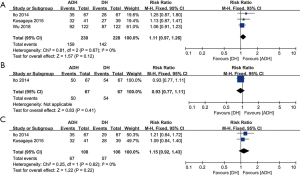
We assessed the efficacy of the NNR between the ADH and DH groups based on the overall NNR, acute NNR, and delayed NNR. Three studies compared the overall NNR (heterogeneity: P=0.67, I2=0%), and the results showed a better NNR in the ADH group, but this difference was not significant (RR: 1.11, 95% CI: 0.97–1.26, P=0.12; Figure 3A). Only one study compared the acute NNR. No significant difference was observed between the two groups (RR: 0.93, 95% CI: 0.77–1.11, P=0.41; Figure 3B). Two studies compared the delayed NNR (heterogeneity: P=0.62, I2=0%). No significant difference was found between the two groups (RR: 1.15, 95% CI: 0.92–1.43, P=0.22; Figure 3C).
Rescue antiemetic treatment
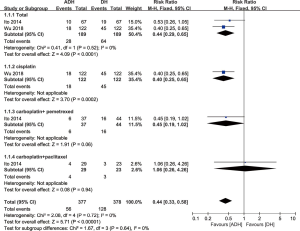
Two studies compared the proportion of patients who received RAT (heterogeneity: P=0.52, I2=0%); A significantly lower number of patients in the ADH group received RAT than patients in the DH group (RR: 0.44, 95% CI: 0.29–0.65, P<0.0001). In the subgroup analysis of chemotherapy regimens, for patients taking cisplatin (RR: 0.40, 95% CI: 0.25–0.65, P=0.0002) and carboplatin + pemetrexed (RR: 0.45, 95% CI: 0.19–1.02, P=0.06), the ADH group showed a significantly lower need for RAT than the DH group. For patients receiving carboplatin + paclitaxel, no significant difference was observed between the two groups (RR: 1.06, 95% CI: 0.26–4.26, P=0.94; Figure 4).
Adverse events
Three studies including 458 patients recorded AEs. We classified the listed AEs in terms of severity (total AEs and grade 3–5 AEs) and type (hematological and nonhematological toxicity) and performed subgroup analyses of the listed AEs.
In the subgroup analysis of total AEs, ADH did not differ from DH in hematological toxicity (leukopenia, neutropenia, anemia, and thrombocytopenia) and nonhematological toxicity (hepatotoxicity, nephrotoxicity, constipation, allergic reaction, hiccups, fatigue, diarrhea, decreased appetite, and abdominal pain; Table S3).
In the subgroup analysis of grade 3–5 AEs, the ADH group did not differ from the DH group in hematological toxicity (leukopenia, neutropenia, anemia, and thrombocytopenia) and nonhematological toxicity (hepatotoxicity, nephrotoxicity, constipation, allergic reaction, hiccups, fatigue, diarrhea, decreased appetite, and abdominal pain; Table S4).
FLIE score
One study adopted the FLIE score to evaluate nausea and vomiting; lower scores indicate better QoL among patients. The median FLIE score (24.97 vs. 38.1, P=0.022), total score greater than 50 (3.2% vs. 31%, P=0.011) and total score greater than 20 (16.1% vs. 44.8%, P=0.015) were compared. Better results were recorded for the ADH group in all three comparisons.
Subgroup analysis
For the primary endpoints, i.e., the CR and NNR, we performed subgroup analyses based on the following classification variables: nation, chemotherapy regimen, 5-HT3 RA, ADC (%) and performance status. A subgroup analysis of the overall CR showed that ADH was superior in some groups stratified by specific clinical features (China, cisplatin-based chemotherapy, 2nd-generation 5-HT3 RA, ADC <50%, and ECOG score of 0–2). In the subgroup analysis of overall NNR, we did not observe significant differences between groups (Table 2).
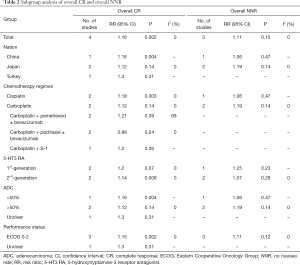
Full table
Sensitivity analysis
No obvious heterogeneity was identified in the analysis of the overall CR and overall NNR. We assessed the stability and sensitivity of the combined result based on degree of influence of individual studies that examined each outcome. The analysis showed that the results related to the CR (Figure S3A) and NNR (Figure S3B) were reliable and stable.
Publication bias
No evidence of publication bias was observed for the overall CR (Begg’s test P=0.734; Egger’s test P=0.961, Figure S4A) and overall NNR (Begg’s test P=0.296; Egger’s test P=0.135, Figure S4B).
Discussion
CINV is the most common complication experienced by patients with LC treated with PBC, and it exerts a significant negative effect on treatment and patients’ QoL. Researchers have not yet clearly determined whether adding aprepitant to DH increases the effectiveness of this treatment. This meta-analysis is the first to compare the effectiveness of ADH and DH among patients with LC receiving PBC based on data from RCTs. Based on our results, the ADH group had a better overall CR and a lower number of patients who needed RAT than the DH group. ADH also showed better trends for the overall NNR and delayed CR. No significant differences in the acute CR, acute NNR, and delayed NNR were observed between the two groups. In the subgroup analysis of the overall CR and overall NNR, ADH was superior in some groups stratified based on specific clinical features (China, cisplatin-based chemotherapy, 2nd-generation 5-HT3 RA, ADC <50%, and ECOG score of 0–2). AEs showed no significant difference in the hematological or nonhematological toxicity.
Our meta-analysis showed that ADH is superior to DH in terms of a better overall CR and overall NNR, as observed in two included RCTs (11,12). Dupuis et al. (20) and Yokoe et al. (21) showed that ADH led to a better CR and NNR. Albany et al. (22) suggested that ADH led to a better CR and NNR, but the differences were not significant. Some studies also showed similar results in patients with different cancers who received different chemotherapy regimens and were of different ages (8,23). We propose two likely explanations for the results: (I) 5-HT3 RA combined with 5-HT3 (a major pathogenic factor in acute CINV) is effective for the acute CR and NNR; aprepitant is an NK-1 RA, mainly for substance P (major pathogenic factor in delayed CINV), which predominates in the delayed phase to improve the CR and NNR. The combination of the two drugs exerts a synergistic effect to prevent CINV in the overall phase (14,20). (II) Aprepitant may be involved in the excretion of 5-HT3 together with central and exerts an antagonistic effect on chemotherapy-induced emetogenic effects (24). By analyzing the secondary endpoints, we also found that the number of patients receiving RAT and the FLIE score (lower means less CINV) were significantly lower in the ADH group than in the DH group, which indirectly indicates that ADH is more effective than DH at preventing CINV. In summary, the analysis of the main indicators and secondary indicators jointly proved that ADH is more effective at preventing CINV than DH and that ADH leads to a better overall CR and overall NNR.
In the subgroup analysis of chemotherapy regimens, different platinum drugs produced different results: the cisplatin subgroup showed a significantly higher overall CR, while the overall CR of the carboplatin subgroup was not significantly different. Two included RCTs (13,14) showed a better CR and NNR in the ADH group, but the differences were not significant. Albany et al. (22) and Miya et al. (8) reported that the ADH group had an effective CR and NNR after treatment with two different chemotherapy regions (cisplatin and carboplatin). We propose several explanations for these findings. Cisplatin-based chemotherapy is regarded as highly emetogenic chemotherapy (HEC), carboplatin-based chemotherapy is regarded as moderately emetogenic chemotherapy (MEC), and the anti-CINV effect of ADH on patients receiving HEC is more obvious (9,10). In summary, our subgroup analysis found that the results may be related to platinum drugs, and ADH has a greater relative advantage in patients receiving HEC. A subgroup analysis of combination therapy showed no significant difference, suggesting that combination therapy had little effect on the CR and NNR. Therefore, ADH was more effective at preventing CINV among patients undergoing HEC (cisplatin, for example). The subgroup of 5-HT3 RA showed that the result was different between 5-HT3 RAs, among which, the second generation 5-HT3 RA was more suitable for ADH than the 1st-generation 5-HT RA. This difference may be due to the factors listed below. (I) With a longer half-life, the 2nd-generation 5-HT3 RA has a longer effective period and it also has a greater receptor binding affinity, resulting higher drug availability (13). (II) The 2nd-generation 5-HT3 RA differentially inhibits NK-1/5-HT3 crosstalk, and thus it exerts a better synergistic effect with aprepitant in the delayed phase (25). In conclusion, we suggested that ADH was more effective at preventing CINV among patients undergoing treatment with HEC (cisplatin, for example) and 2nd-generation 5-HT3 RA.
In the subgroup analysis of AEs, no significant difference was observed in hematological toxicity and nonhematological toxicity. Among AEs classified as hematological toxicity, the most common complications were leukopenia, neutropenia, anemia and thrombocytopenia. Among AEs classified as nonhematological toxicity, the most common complications were hepatotoxicity, constipation, hiccups, fatigue and decreased appetite. Hashimoto et al. (26) and Pasricha et al. (27) did not observe significant differences in patients treated with ADH and DH. The most commonly reported AEs of aprepitant were asthenia/fatigue, hiccups, constipation, diarrhea and anorexia (28,29). Our results showed no significant difference among these AEs. Some articles reported that aprepitant may be secondary to anaphylactic shock and cardiac arrest (30). One of our included RCTs also reported one case of anaphylactic shock, but no significant difference was found (14); however, additional high-quality RCTs are necessary to analyze and confirm this conclusion. In general, the subgroup analysis of AEs showed no significant difference between the ADH and DH groups, confirming that ADH achieved similar safety to that of DH.
The analysis still has some limitations that would affect the results. On the one hand, although the four included articles were all high-quality RCTs, the reliability of the outcomes might be affected by the limited numbers of articles and patients. On the other hand, all articles were published in English, and language bias may exist. Moreover, the applicability of the results may be affected by the fact that the data we collected were obtained from Asian patients residing in three nations (China, Japan, and Turkey). In addition, different doses or types of 5-HT3 RAs and dexamethasone may affect the accuracy of the results. Furthermore, the applicability of the results may be reduced because the obtained patients were mainly middle-aged and elderly people, which may be related to the incidence of LC. Last but not least, the articles did not specify the type of LC, and thus the representativeness of the results may be influenced. These limitations should be used to guide future research.
Conclusions
Compared with DH, ADH appears to be superior for patients with LC who are treated with PBC in terms of a CR for CINV. Subgroup analyses showed advantages in some groups with specific clinical features (China, cisplatin-based chemotherapy, 2nd-generation 5-HT3 RA, ADC <50%, and ECOG score of 0-2). No significant difference was observed in the hematological toxicity and non-hematological toxicity between total AEs and grade 3–5 AEs. However, because of the potential deficiency of our meta-analysis, more large-scale, high-quality RCTs are required to verify this conclusion.
Acknowledgments
The authors thank professor Jianjun Xu, MD (Department of Thoracic Surgery, The Second Affiliated Hospital of Nanchang University) for his statistical advice. We also appreciate the company of AJE for checking all wordings of the main text, figures and tables.
Funding: This study was supported by National Natural Science Foundation of China (NSFC), number of grants (81560345), Natural Science Foundation of Jiangxi Province (Grant number: 20181BAB215027). The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2290
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-2290
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2290). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Kalemkerian GP, Loo BW, Akerley W, et al. NCCN Guidelines Insights: Small Cell Lung Cancer, Version 2.2018. J Natl Compr Canc Netw 2018;16:1171-82. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Grimison P, Mersiades A, Kirby A, et al. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: a randomised, placebo-controlled, phase II crossover trial. Ann Oncol 2020;31:1553-60. [Crossref] [PubMed]
- Sommariva S, Pongiglione B, Tarricone R. Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: A systematic review. Crit Rev Oncol Hematol 2016;99:13-36. [Crossref] [PubMed]
- Navari RM, Schwartzberg LS. Evolving role of neurokinin 1-receptor antagonists for chemotherapy-induced nausea and vomiting. Onco Targets Ther 2018;11:6459-78. [Crossref] [PubMed]
- Miya T, Kobayashi K, Hino M, et al. Efficacy of triple antiemetic therapy (palonosetron, dexamethasone, aprepitant) for chemotherapy-induced nausea and vomiting in patients receiving carboplatin-based, moderately emetogenic chemotherapy. Springerplus 2016;5:2080. [Crossref] [PubMed]
- Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: ASCO Guideline Update. J Clin Oncol 2020;38:2782-97. [Crossref] [PubMed]
- Berger MJ, Ettinger DS, Aston J, et al. NCCN Guidelines Insights: Antiemesis, Version 2.2017. J Natl Compr Canc Netw 2017;15:883-93. [Crossref] [PubMed]
- Wu F, Lin X, Yang Z, et al. Phase III Randomized Trial of Palonosetron and Dexamethasone With or Without Aprepitant to Prevent Nausea and Vomiting Induced by Full-dose Single-day Cisplatin-based Chemotherapy in Lung Cancer. Clin Lung Cancer 2018;19:e913-8. [Crossref] [PubMed]
- Aksu G, Dolasik I, Ensaroglu F, et al. Evaluation of the efficacy of aprepitant on the prevention of chemotherapy-induced nausea and vomiting and quality of life with functional living index emesis. Balkan Med J 2013;30:64-7. [Crossref] [PubMed]
- Kusagaya H, Inui N, Karayama M, et al. Evaluation of palonosetron and dexamethasone with or without aprepitant to prevent carboplatin-induced nausea and vomiting in patients with advanced non-small-cell lung cancer. Lung Cancer 2015;90:410-6. [Crossref] [PubMed]
- Ito Y, Karayama M, Inui N, et al. Aprepitant in patients with advanced non-small-cell lung cancer receiving carboplatin-based chemotherapy. Lung Cancer 2014;84:259-64. [Crossref] [PubMed]
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380-2. [Crossref] [PubMed]
- Dupuis LL, Tomlinson GA, Pong A, et al. Factors Associated With Chemotherapy-Induced Vomiting Control in Pediatric Patients Receiving Moderately or Highly Emetogenic Chemotherapy: A Pooled Analysis. J Clin Oncol 2020;38:2499-509. [Crossref] [PubMed]
- Yokoe T, Hayashida T, Nagayama A, et al. Effectiveness of Antiemetic Regimens for Highly Emetogenic Chemotherapy-Induced Nausea and Vomiting: A Systematic Review and Network Meta-Analysis. Oncologist 2019;24:e347-57. [Crossref] [PubMed]
- Albany C, Brames MJ, Fausel C, et al. Randomized, Double-Blind, Placebo-Controlled, Phase III Cross-Over Study Evaluating the Oral Neurokinin-1 Antagonist Aprepitant in Combination With a 5HT3 Receptor Antagonist and Dexamethasone in Patients With Germ Cell Tumors Receiving 5-Day Cisplatin Combination Chemotherapy Regimens: A Hoosier Oncology Group Study. J Clin Oncol 2012;30:3998-4003. [Crossref] [PubMed]
- Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N Engl J Med 2016;375:134-42. [Crossref] [PubMed]
- Chen S, Lu M, Liu D, et al. Human substance P receptor binding mode of the antagonist drug aprepitant by NMR and crystallography. Nat Commun 2019;10:638. [Crossref] [PubMed]
- Rojas C, Li Y, Zhang J, et al. The antiemetic 5-HT3 receptor antagonist Palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther 2010;335:362-8. [Crossref] [PubMed]
- Hashimoto H, Abe M, Tokuyama O, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:242-9. [Crossref] [PubMed]
- Pasricha PJ, Yates KP, Sarosiek I, et al. Aprepitant Has Mixed Effects on Nausea and Reduces Other Symptoms in Patients With Gastroparesis and Related Disorders. Gastroenterology 2018;154:65-76.e11. [Crossref] [PubMed]
- Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer 2017;109:58-61. [Crossref] [PubMed]
- Muñoz M, Rosso M, Coveñas R. Neurokinin-1 Receptor Antagonists against Hepatoblastoma. Cancers (Basel) 2019;11:1258. [Crossref] [PubMed]
- Schwartzberg L, Roeland E, Andric Z, et al. Phase III safety study of intravenous NEPA: a novel fixed antiemetic combination of fosnetupitant and palonosetron in patients receiving highly emetogenic chemotherapy. Ann Oncol 2018;29:1535-40. [Crossref] [PubMed]



