
New application of direct lymphangiography in the diagnosis and treatment of chylothorax after lung cancer surgery: a case series
Introduction
Chylothorax is a rare but relatively severe complication of lung cancer surgery. It accounts for about 1–2% of secondary chylothorax after classic lung resection and post-mediastinal lymph node dissection (MLND) (1-3). Although secondary chylothorax after lung cancer surgery is not common, it is a potentially life-threatening complication and sometimes difficult to treat. Common treatments include low-fat diet, total parenteral nutrition (TPN), medications, pleurodesis, and so on. These treatments sometimes do not work (4), especially in cases of high-output of chyle leak. In previous literatures, direct lymphangiography (DLG) was used to diagnose intra-thoracic lymphatic leaks after surgery. Based on this, thoracotomy, thoracoscopy, and interventional methods were used as treatment to ligate or embolize the thoracic duct (5,6). Through analysis of DLG of more than 3,000 patients with chylous effusion in the past, we have identified a common phenomenon of thoracic duct outlet obstruction and thoracic duct dilatation. Among the 4 cases of chylothorax secondary to lung cancer surgery reported in this study, various degrees of thoracic duct obstruction and thoracic duct dilatation were also found in DLG. Thoracic ductoplasty was performed on 3 participants to reduce lymphatic circulation pressure. Satisfactory treatment outcome is reported as follows.
We present the following article in accordance with the MDAR and AME Case Series reporting checklist (available at http://dx.doi.org/10.21037/apm-21-263).
Methods
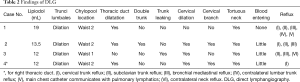
A retrospective analysis was conducted on 4 patients in Department of Lymphatic Surgery, Beijing Shijitan Hospital, Capital Medical University, who with secondary chylothorax after lung cancer surgery, including 3 males and 1 female. The average age was 58 years (47–64 years). Lung squamous cell carcinoma was the primary disease in 3 participants, and 1 had lung adenocarcinoma. Of the 4 cases, 1 involved a tumor located in the left lung and 3 in the right lung. All participants underwent lobectomy and MLND, after which milky white pleural effusion ensued—3 cases of unilateral pleural effusion and 1 case of bilateral pleural effusion. All participants then underwent conservative treatment including low-fat diet, fasting, and TPN, which proved ineffective. The nature of the pleural drainage was determined by appearance and triglyceride test (see Table 1 for details). To further determine the causes, the 4 patients underwent direct lymphangiography.

Full table
The direct lymphangiography (DLG) is a selective imaging method of the lymphatic system using iodized oil. It was first reported by Kinmonth in 1952 (7). There are two main surgical approaches to lymphangiography: dorsal foot lymphatic aspiration and direct lymph node aspiration. Both of these techniques have advantages and disadvantages, and the appropriate surgical site needs to be selected according to clinical indications and surgeon’s preference. The cases involved in this paper were lymphangiography via dorsal foot lymphatic puncture. This method involves intradermal injection of a mixture of blue dye [methylene blue injection (Jichuan Pharmaceutical, Taixing, Jiangsu, China)] and lidocaine hydrochloride into the web space between the first and second toe (Suicheng Pharmaceutical, Tianjin, China) 2 mL. After injection of 1 mL, the superficial lymphatic vessels at the arch of the foot can be identified macroscopically. Upon local anesthesia, the blue-stained lymphatic vessels were dissected, punctured with lymphangiography needles (Cook Medical, Bloomington, IN, USA), and injected with lipiodol (Guerbet, Roissy, France) (recommended maximum injection volume is 20 mL at a speed of 6–8 mL/h). Subsequently, the imaging mode of digital subtraction angiography (DSA) was used for dynamic inspection (6). All 4 participants underwent DLG via a dorsal right foot approach, with an average injection of 14.1 mL of lipiodol. The lipiodol went up through the dorsal foot lymphatic vessels, passed through the lower extremity lymphatic vessels, lumbar trunk, chyle pool, thoracic duct, and finally flowed into the neck vessels. The morphology, function, and abnormalities of lymphatic vessels were recorded in detail.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). The study was approved by Chinese Clinical Trial Registry (No.: ChiCTR-DPC-14005464), and informed consent was taken from all the patients.
Results
Thoracic duct outlet obstruction and contrast backflow were common in the lymphangiography of the 4 participants, as shown in Figures 1-4 and Table 2. Case 1 was previously treated with thoracic duct ligation (TDL); however, on DLG, the thoracic duct could be seen throughout its course, and no double-thoracic duct trunk structure was found. As a result, it was concluded that previous TDL had been unsuccessful. For Case 3, lymphangiogram revealed a large amount of contrast agent leaking out the thoracic duct and entering the right thoracic cavity. Case 4 was a participant with a right thoracic duct.
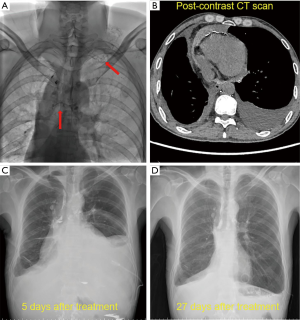

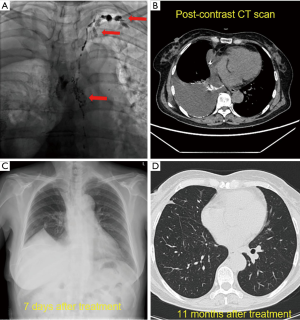
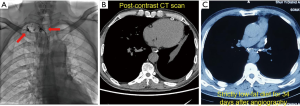
Full table
Thoracic ductoplasty was performed on 3 participants, which alleviated obstruction of the chylous recirculation pathway and reduced the pressure of lymphatic circulation. All 3 were then treated with strict low-fat diet after surgery. The remaining participant received a strict low-fat diet (fat ratio <2%), only without surgery. Long-term follow-up was undertaken, the longest being 20 months. Chest imaging of these 4 participants revealed disappearance of pleural effusion.
Discussion
Classical lung resection and MLND dominate the surgical treatment of lung cancer. Chylothorax is a rare but relatively serious sequela of lung cancer surgery, with an incidence of 1–2% (1-3). Site, type of resection, surgical technique, histology, and staging of lung cancer have no effect on the occurrence of postoperative chylothorax (8). Due to the anatomical position of the thoracic duct within the thorax, MLND is very likely to damage the thoracic duct and its branches, which is the cause of chyle leak. The leakage of chyle can lead to malnutrition, immunosuppression, and dyspnea, which threatens patients' lives and requires further treatment (9). Common clinical treatment methods are classified as either conservative or surgical. Conservative treatments are mainly medications, low-fat diet, fasting, TPN, pleurodesis, and so on. Surgical methods include TDL and thoracic duct embolization (TDE) (10).
In conservative treatment protocols, multiple approaches are often utilized simultaneously. In a study of 41 postoperative patients with chylothorax, Bryant et al. (2) injected 200 mg of somatostatin subcutaneously every 8 hours while the participants were fasting, and the drainage decreased to 450 mL/day after 48 hours. The participants were then given a medium chain triglyceride (MCT) diet for another 48 hours. If the drainage remained below 450 mL/day, the chest drain would be removed and the MCT diet continued for 2 weeks. This protocol allowed 90% of patients to be cured, whereas another 10% of patients underwent TDL and pleurodesis. This result was consistent with that of another group of studies where the cure rate of postoperative chylothorax by low-fat diet and pleurodesis was 84%, and 16% required TDL (11). Octreotide lowers concentration of triglycerides in the thoracic duct by reducing absorption of fat from the small intestine (12), thereby reducing the rate of chyle leak. A recent study reported that octreotide was only effective in 47% of chylothorax cases (13). Furthermore, prophylactic use of octreotide has not been shown to decrease incidence of chylothorax after surgery (14).
For patients with high output chylothorax (>1,000 mL/24 h), conservative treatment is usually unsuccessful, and early surgical intervention can significantly reduce mortality of those patients who show no improvement after conservative treatment (15). Finding and repairing chyle leaks is the ideal solution to chylothorax, but is difficult to achieve in practice (4). The most popular procedure is TDL, with thoracic duct exploration during surgery (4); however, the repeated failure of TDL is perplexing (16). According to Johnson et al., 40–60% of thoracic ducts have anatomical variations (17). Although butter, cream, methylene blue, and other agents can be used to aid recognition of thoracic ducts, these are not ideal solutions in practice. Recently, some researchers have reported that the use of indocyanine green (ICG) near-infrared fluorescence imaging can assist in identifying thoracic ducts during TDL (18). In our study, DLG confirmed that the TDL was unsuccessful. In addition, a right thoracic duct was detected in Case 4. The therapeutic effect of TDE, although a novel therapy, is equivalent to TDL (19), and has the most notable advantage of being minimally invasive. Nevertheless, the TDE operation is extremely difficult despite being considered a minor procedure. Pamarthi et al. (20) conducted a retrospective study of 105 patients with idiopathic and postoperative chylothorax who underwent TDE. The success rate was 79%, with difficulty of chylotomy being the major cause of failure of this procedure.
The TDE operation is difficult, and technical success does not equate to a successful treatment (21). It has been reported that TDL is curative in 90% of chylothorax cases (22). In light of this, finding a more effective diagnosis and treatment plan for chylothorax secondary to lung cancer surgery may bring new hope for patients with severe chylothorax. Historically, DLG has been employed in the diagnosis and treatment of chylothorax, and has even been performed routinely. Moreover, some studies have reported frequent application of lymphangiography to search for intrathoracic leaks and subsequent CT scans to improve diagnostic accuracy (23). In our clinical practice, DLG is also routinely performed to assist in the diagnosis and treatment of chylothorax; meanwhile, the overall function of the patient's lymphatic system, including whether there is intrathoracic leak, is closely monitored. Of the 4 cases reported in this study, 3 (75%) were not found to have iodized oil entering the thoracic cavity on angiography, and 1 (25%) had iodized oil entering the right thoracic cavity. All 4 cases (100%) were observed to have catheter dilation and impediments to iodized oil entering the bloodstream at the end of the thoracic duct. These imaging features are consistent with thoracic duct outlet obstruction.
Normal adults produce about 2–4 L of lymphatic fluid daily, 90% of which returns to the bloodstream through the thoracic duct; obstruction of the thoracic duct outlet will inevitably increase the pressure of the lymphatic system, leading to a series of pathological changes. Various contrast backflow phenomena found in lymphangiography are caused by elevated pressure in the lymphatic circulation due to thoracic duct outlet obstruction and valvular insufficiency in the thoracic duct, which result in backflow of contrast into other major lymphatic branches connected with the thoracic duct (24). The types of lymphatic backflow include cervical branch, subclavian branch, bronchomediastinal branch, pulmonary branch, and contralateral lumbar branch backflow. The lymphatic fluid in these returning lymphatic vessels should have been otherwise introduced into the thoracic duct. The emergence of lymphatic reflux is evidence of increased pressure in the thoracic duct. On the assumption that the thoracic duct outlet is obstructed and the pressure is increased, if the thoracic branches or the branches of the thoracic duct are iatrogenically damaged, a large amount of chyle-containing lymphatic fluid will leak out. Due to the negative pressure in the thoracic cavity, which is significantly different from the pressure in the thoracic duct, the amount of leak may be prominent. Moreover, continuous chest tube drainage keeps this mechanism of chyle leak working, which is why patients with thoracic duct outlet obstruction tend to have high output chylothorax after lung cancer surgery.
In our clinical work, thoracic ductoplasty is performed to unblock the returning channel of chyle-containing lymphatic fluid, thereby decreasing the pressure in the thoracic duct, reducing amount of chyle leak, and facilitating the healing of the leak. Case 4 effectively reduced chyle production by strictly limiting fat intake, leading to decreased pressure in the lymphatic circulation. Our theory can explain why TDL and TDE were unsuccessful in patients with chylothorax secondary to lung cancer surgery. The pressure in the lymphatic system rises due to the patient's lymphatic return being obstructed. Ligation or embolization of the main branch of the thoracic duct further elevates lymphatic circulation pressure. Consequently, TDL and TDE play no therapeutic role for patients with secondary chylothorax, and even worse, these procedures may aggravate their conditions. Kiang (25) performed a direct contrast-enhanced magnetic resonance lymphangiography of a chylothorax patient with TDE failure. It was found that chyle entered the thoracic cavity through the retroperitoneal space after TDE, and the thoracic duct exhibited prominent dilation after the procedure, which is consistent with our theory. Compared with TDL, thoracotomy, and thoracoscopy, thoracic duct exploration is much less invasive, thus patients usually experience less pain postoperatively. Additionally, thoracic ductoplasty restores normal anatomy and physiology of the human lymphatic system rather than irreversibly blocking lymphatic return as in TDL and TDE. Therefore, no matter if it is from the point of minimizing the length of incision, or from the perspective of restoring normal anatomy and physiology, our approach to treating chylothorax secondary to lung cancer surgery was shown to be more superior—the lymphatic reflux status of the patient is first evaluated by DLG, followed by thoracic ductoplasty with a small incision. For the diagnosis and treatment of chylothorax after lung cancer surgery, we recommend conservative treatment first. If conservative treatment fails, DLG should be performed as soon as possible to assess the patient’s lymphatic circulation. If thoracic duct outlet obstruction is evident upon DLG, thoracic duct reconstruction should be performed. Hopefully our approach can shed some light on diagnosis and treatment of chylothorax secondary to lung cancer surgery.
Acknowledgments
The authors thank the patients who participated in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR and AME Case Series reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-263
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-263
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-263). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). The study was approved by Chinese Clinical Trial Registry (No.: ChiCTR-DPC-14005464), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cho HJ, Kim DK, Lee GD, et al. Chylothorax complicating pulmonary resection for lung cancer: effective management and pleurodesis. Ann Thorac Surg 2014;97:408-13. [Crossref] [PubMed]
- Bryant AS, Minnich DJ, Wei B, et al. The incidence and management of postoperative chylothorax after pulmonary resection and thoracic mediastinal lymph node dissection. Ann Thorac Surg 2014;98:232-5; discussion 235-7. [Crossref] [PubMed]
- Liu CY, Hsu PK, Huang CS, et al. Chylothorax complicating video-assisted thoracoscopic surgery for non-small cell lung cancer. World J Surg 2014;38:2875-81. [Crossref] [PubMed]
- Shah RD, Luketich JD, Schuchert MJ, et al. Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg 2012;93:897-903; discussion 903-4. [Crossref] [PubMed]
- Kaschner GM, Strunk H. Transnodal ultrasound-guided lymphangiography for thoracic duct embolization in chylothorax. Dtsch Med Wochenschr 2014;139:2231-6. [Crossref] [PubMed]
- Boffa DJ, Sands MJ, Rice TW, et al. A critical evaluation of a percutaneous diagnostic and treatment strategy for chylothorax after thoracic surgery. Eur J Cardiothorac Surg 2008;33:435-9. [Crossref] [PubMed]
- Kinmonth JB. Lymphangiography in man; a method of outlining lymphatic trunks at operation. Clin Sci 1952;11:13-20. [PubMed]
- Kutlu CA, Sayar A, Olgac G, et al. Chylothorax: a complication following lung resection in patients with NSCLC - chylothorax following lung resection. Thorac Cardiovasc Surg 2003;51:342-5. [Crossref] [PubMed]
- McGrath EE, Blades Z, Anderson PB. Chylothorax: Aetiology, diagnosis and therapeutic options. Respir Med 2010;104:1-8. [Crossref] [PubMed]
- Bender B, Murthy V, Chamberlain RS. The changing management of chylothorax in the modern era. Eur J Cardiothorac Surg 2016;49:18-24. [Crossref] [PubMed]
- Takuwa T, Yoshida J, Ono S, et al. Low-fat diet management strategy for chylothorax after pulmonary resection and lymph node dissection for primary lung cancer. J Thorac Cardiovasc Surg 2013;146:571-4. [Crossref] [PubMed]
- Bello SO, Rahamim J. High-dose intravenous octreotide is safe and may be superior to surgery in managing severe postesophagectomy chylothorax in high-risk patients. Ann Thorac Surg 2015;100:297-9. [Crossref] [PubMed]
- Bellini C, Cabano R, De Angelis LC, et al. Octreotide for congenital and acquired chylothorax in newborns: A systematic review. J Paediatr Child Health 2018;54:840-7. [Crossref] [PubMed]
- Zhang C, Zhang H, Wu W, et al. Prophylactic octreotide does not reduce the incidence of postoperative chylothorax following lobectomy: Results from a retrospective study. Medicine (Baltimore) 2019;98:e16599 [Crossref] [PubMed]
- Merigliano S, Molena D, Ruol A, et al. Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. J Thorac Cardiovasc Surg 2000;119:453-7. [Crossref] [PubMed]
- Bédat B, Scarpa CR, Sadowski SM, et al. Acute pancreatitis after thoracic duct ligation for iatrogenic chylothorax. A case report. BMC Surg 2017;17:9. [Crossref] [PubMed]
- Johnson OW, Chick JF, Chauhan NR, et al. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol 2016;26:2482-93. [Crossref] [PubMed]
- Yang F, Zhou J, Li H, et al. Near-infrared fluorescence-guided thoracoscopic surgical intervention for postoperative chylothorax. Interact Cardiovasc Thorac Surg 2018;26:171-5. [Crossref] [PubMed]
- Schild HH, Naehle CP, Wilhelm KE, et al. Lymphatic Interventions for Treatment of Chylothorax. Rofo 2015;187:584-8. [Crossref] [PubMed]
- Pamarthi V, Stecker MS, Schenker MP, et al. Thoracic duct embolization and disruption for treatment of chylous effusions: experience with 105 patients. J Vasc Interv Radiol 2014;25:1398-404. [Crossref] [PubMed]
- Yannes M, Shin D, McCluskey K, et al. Comparative Analysis of Intranodal Lymphangiography with Percutaneous Intervention for Postsurgical Chylous Effusions. J Vasc Interv Radiol 2017;28:704-11. [Crossref] [PubMed]
- Martucci N, Tracey M, Rocco G. Postoperative Chylothorax. Thorac Surg Clin 2015;25:523-8. [Crossref] [PubMed]
- Deso S, Ludwig B, Kabutey NK, et al. Lymphangiography in the diagnosis and localization of various chyle leaks. Cardiovasc Intervent Radiol 2012;35:117-26. [Crossref] [PubMed]
- Melduni RM, Oh JK, Bunch TJ, et al. Reconstruction of occluded thoracic duct for treatment of chylopericardium: a novel surgical therapy. J Vasc Surg 2008;48:1600-2. [Crossref] [PubMed]
- Kiang SC, Ahmed KA, Barnes S, et al. Direct contrast-enhanced magnetic resonance lymphangiography in the diagnosis of persistent occult chylous effusion leak after thoracic duct embolization. J Vasc Surg 2019;7:251-7. [PubMed]
(English Language Editor: J. Jones)


