
A narrative review of the impact of cerebellar dysfunction and sleep disturbances after general anesthesia in patients with Alzheimer’s disease
Background
Alzheimer’s disease (AD) is one of the main causes of dementia in the senium and presenium, which is clinically characterized by memory loss, decreased intelligence and loss of fine motor skills. The brains of AD patients have obvious characteristics of atrophy, most notably in the temporal and parietal lobes (1). Early histopathological studies of AD in humans focused on the cerebrum, and the cerebellum was thought to be unrelated to AD. However, accumulating evidence has revealed several pathological changes in the cerebellum in early-onset AD (2,3).
Moreover, sleep is also important to central nervous system physiology. Many studies have shown that sleep disorders are risk factors for AD. Notably, patients with AD exhibit an increased frequency of sleep disorders (4,5). General anesthesia is widely used in the clinical setting, and the loss of consciousness induced by general anesthetics is accompanied by a gradual decrease of the subject’s ability to perceive the external environment. Several studies have shown that anesthesia may be neurotoxic and cause various long-term behavioral disorders, which have been reported to increase the risk of AD (6-8).
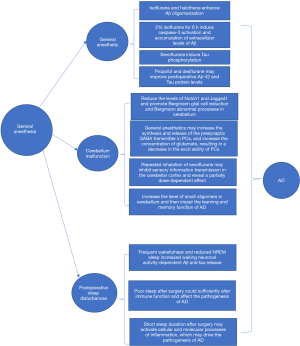
We aimed to review and summarize information regarding the following three questions (Figure 1): (I) how does general anesthesia affect AD-related proteins? (II) how does general anesthesia affect AD by influencing cerebellar function? and (III) how does postoperative sleep disturbance after general anesthesia aggravate AD?
We present the paper in accordance with the Narrative Review reporting checklist (available at: http://dx.doi.org/10.21037/apm-20-2597).
Effect of general anesthesia on AD-related proteins
Recent pre-clinical and clinical studies support the notion that general anesthetics have a notable impact on tau pathogenesis and amyloid-β (Aβ) peptide, which could contribute to the development of AD (9,10). Eckenhoff et al. proved that inhalation anesthetics isoflurane and halothane can enhance Aβ oligomerization and Aβ-induced cytotoxicity of rat pheochromocytoma cells in vitro (11). Xie et al. reported that treatment with 2% isoflurane for 6 hours can induce caspase-3 activation, cell death, and accumulation of extracellular Aβ levels (12). However, desflurane is a new type of inhalation anesthetic, different from isoflurane and sevoflurane, it has no effect on the activation of caspase-3, the processing of Aβ precursor protein and the accumulation of Aβ in human glioma cells, and it does not induce learning and memory impairments in mice (13). Zhen et al. (14) showed that nitrous oxide does not cause apoptosis or Aβ accumulation in cells and neurons, as pointed out by desflurane. These findings indicate that compared with other commonly used inhalation anesthetics (such as isoflurane and sevoflurane), the effects of nitrous oxide and desflurane on Aβ protein have better characteristics (10).
A study showed that propofol did not affect Aβ precursor protein unlike the inhalation anesthetics isoflurane and sevoflurane. Propofol was also found to inhibit isoflurane-induced Aβ42 oligomerization (15). Fodale et al. confirmed that smaller-sized agents like volatile anesthetics can promote Aβ oligomerization, while larger-sized intravenous agents cannot interact with Aβ protein and do not promote Aβ oligomerization (halothane > isoflurane > sevoflurane > propofol > thiopental > diazepam) (16).
Tau is a phosphoprotein, and its biological activity of stimulating microtubule assembly is regulated by phosphorylation. Run et al. demonstrated that the abnormal hyperphosphorylation and aggregation of tau were crucial to neurodegeneration in AD (17). Previous studies showed that sevoflurane anesthesia induced tau phosphorylation and cognitive impairment in 6-day-old neonatal mice (18,19). Compared with sevoflurane, propofol may improve postoperative Aβ-42 and tau protein levels in patients with hepatocellular carcinoma and ameliorate postoperative cognitive function (20).
Effect of cerebellar dysfunction after general anesthesia on AD
The cerebellum is a key part of distributed neural circuits, not only involved in motor functions, but also in autonomic nervous, limbic and cognitive behaviors. Motor cerebellar lesions can cause movement disorders, but cognitive and limbic cerebellar lesions in the posterior lobe can cause intellectual and emotional sensory disorders (cerebellar affective syndrome) (1). Purkinje cells (PC) are the only output neurons in the cerebellar cortex and are highly sensitive to anesthetics (21). Repeated anesthesia may cause PC degeneration, dendritic cell reduction and cerebellar degeneration (22). Several studies have suggested that cerebellar dysfunction contributes to the development of AD, including the loss of distal dendritic segments, decrease in the total number of dendritic spines, presence of ubiquitin-immunoreactive dystrophic neurites and spines, microglial proliferation of PCs, and significant cell volume loss. Aβ deposits in the cerebellum are also a frequent finding in early-onset AD, and they are located predominantly in the molecular layer of the cerebellar cortex; they are characterized by diffuse-type Aβ with only few amyloid fibrils and generally do not include senile plaques (23-25).
Cerebellar dysfunction caused by general anesthesia is an important factor in promoting AD. General anesthetics may increase the synthesis and release of the presynaptic GABA transmitter in PC, which in turn increases the concentration of glutamate and reduces the excitability of PC (26). Propofol works by activating GABA-A receptors and blocking N-methyl-D-aspartate (NMDA) glutamate receptors (27) and it is a well-known cause of dyskinesias, which strongly highlights the mechanism underlying propofol damage of the cerebellum. Zhang et al. pointed out that the administration of propofol may affect information processing through the climbing fiber-PC pathway through NMDA receptors, which may be related to the propofol-induced dysfunction of the cerebellum, such as in movement disorders (28). Bergman glial cells with filamentous burls extend longer radial fibers in synchronization with the growth of PC dendrites during postpartum development, and contribute to the growth of cerebellar PC dendrites (29-31). In addition, Lütolf et al. reported that the Notch signaling pathway is an important factor in the differentiation and maturation of Bergmann glial cells (32). However, even at low doses, propofol treatment reduces the levels of Notch1 and Jagged1, which promotes Bergmann glial cell reduction and Bergmann abnormal processes, further slowing down granule cell migration and weakening of cerebellar PC development (33). Fang et al. noted that the inhalation of sevoflurane seemed to inhibit sensory information transmission in the cerebellar cortex and had a partially dose-dependent effect, further delaying cerebellar motor function development (34). Moreover, inhaled anesthetics may also increase the level of small oligomers in the cerebellum (35), such as Aβ peptide aggregates, and impair learning and memory function in AD.
Effect of postoperative sleep disturbances after general anesthesia on AD
General anesthesia is considered to be an independent risk factor for circadian rhythm disorders, which may change the sleep structure and quality of sleep after surgery (36). Patients usually experience circadian rhythm disturbances after surgery, such as sleep deprivation and sleep disorders, which are characterized by suppression of slow wave sleep and rapid eye movement sleep during the first three nights after surgery (37). In approximately 23% of patients, the decline in sleep quality may persist for 4 days after surgery. About 25% of patients reported experiencing sleep deprivation again 15 days after surgery, and 24% of them required medication to improve sleep (38). Long-term sleep disturbances may cause central nervous system disorders and memory impairment (39,40). AD is the most prevalent neurodegenerative disorder, and a widely held clinical view is that AD is associated with an increased frequency of sleep disturbances. Sleep disturbances might heighten the risk of AD by increasing Aβ burden (41), and sleep disturbance may also lead to increased systemic inflammation, which is strongly considered to contribute to the Aβ burden which drives AD pathogenesis (42,43).
The following are the potential mechanisms underlying the association between sleep disturbance and AD. First, slow-wave activity during non-rapid eye movement sleep causes decreased neuronal activity, thereby preventing the increase in extracellular Aβ levels and the formation of amyloid plaques (4). Extracellular Aβ levels are closely related to neuronal firing and frequent wakefulness (44). Therefore, postoperative sleep disturbance may lead to increased neuronal activity-dependent Aβ and tau release during awakening, leading to larger deposits of amyloid plaques and tau tangles. In addition, pathological proteins lead to uncoupling of lactate metabolism and neurometabolism in the sleep-wake cycle, leading to severe sleep disorders after surgery. Second, sleep has well-documented effects on the immune system, in that sleep disturbances may modulate optimal immune function and certain cytokines or other immune modulators may enhance or suppress sleep. Therefore, it is conceivable that lack of sleep after surgery will fully change the immune function and affect the pathogenesis of AD (45). Third, short sleep duration after surgery may activate the cellular and molecular processes of inflammation, including increases in nuclear factor-κ-B signaling, which leads to inflammatory gene expression, the production of proinflammatory cytokines, and systemic inflammation with further increases of proinflammatory cytokines and C-reactive protein (46,47). The accumulation of Aβ peptide is crucial in the pathogenesis of AD, and this accumulation is partly driven by inflammation (48). Moreover, in humans, sleep is known to contribute to Aβ clearance from the brain; postoperative sleep disturbances may decrease the clearance of Aβ and contribute to the pathogenesis of AD (49).
Conclusions
This review not only highlighted the effect of general anesthetics on Aβ and tau in AD, but also summarized the effect of cerebellar dysfunction caused by general anesthesia on AD. Moreover, we discussed the mechanism underlying the effect of postoperative sleep disturbances on AD. Pathological changes in the cerebellum are related to early-onset AD. Further, sleep disturbances are frequent in AD and have significant impact on patients and caregivers. Further research is needed to clarify the contribution of general anesthesia to sleep impairment and cerebellar dysfunction. Identifying early cerebellar dysfunction after general anesthesia and developing new therapeutic measures that target postoperative sleep disturbances may have far-reaching implications for AD.
Acknowledgments
The authors would like to thank Raymond C. Koehler, MD, PhD, from the Department of Anesthesiology and Critical Care Medicine, Johns Hopkins, University, Baltimore, MD, USA and Dr. Weifeng Song, MD, PhD, from the Department of Anesthesiology and Perioperative Medicine, School of Medicine, the University of Alabama at Birmingham, Birmingham, Alabama, USA for their discussion and advice on this study.
Funding: The present study was funded by the Joint plan of key R&D plan of Liaoning Provincial Science and Technology Department (2020JH2/10300123), 345 Talent project and the Support Plan for Innovative Talents in Liaoning Higher Education Institution (grant No. 201834).
Footnote
Reporting Checklist: We have completed the Narrative Review reporting checklist. available at: http://dx.doi.org/10.21037/apm-20-2597
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/apm-20-2597). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mavroudis I, Petridis F, Kazis D, et al. Purkinje Cells Pathology in Alzheimer's Disease. Am J Alzheimers Dis Other Demen 2019;34:439-49. [Crossref] [PubMed]
- Mavroudis I. Cerebellar pathology in Alzheimer's disease. Hell J Nucl Med 2019;22:174-9. [PubMed]
- Liang KJ, Carlson E. Resistance, vulnerability and resilience: A review of the cognitive cerebellum in aging and neurodegenerative diseases. Neurobiol Learn Mem 2020;170:106981 [Crossref] [PubMed]
- Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology: a bidirectional relationship. Nat Rev Neurol 2014;10:115-9. [Crossref] [PubMed]
- Borges CR, Poyares D, Piovezan R, et al. Alzheimer's disease and sleep disturbances: a review. Arq Neuropsiquiatr 2019;77:815-24. [Crossref] [PubMed]
- Reddy SV. Effect of general anesthetics on the developing brain. J Anaesthesiol Clin Pharmacol 2012;28:6-10. [Crossref] [PubMed]
- Sinner B, Becke K, Engelhard K. General anaesthetics and the developing brain: an overview. Anaesthesia 2014;69:1009-22. [Crossref] [PubMed]
- Inan G, Özköse Satirlar Z. Alzheimer disease and anesthesia. Turk J Med Sci 2015;45:1026-33. [Crossref] [PubMed]
- Whittington RA, Bretteville A, Dickler MF, et al. Anesthesia and tau pathology. Prog Neuropsychopharmacol Biol Psychiatry 2013;47:147-55. [Crossref] [PubMed]
- Xie Z, Xu Z. General anesthetics and β-amyloid protein. Prog Neuropsychopharmacol Biol Psychiatry 2013;47:140-6. [Crossref] [PubMed]
- Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology 2004;101:703-9. [Crossref] [PubMed]
- Xie Z, Culley D, Dong Y, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol 2008;64:618-27. [Crossref] [PubMed]
- Zhang Y, Xu Z, Wang H, et al. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 2012;71:687-98. [Crossref] [PubMed]
- Zhen Y, Dong Y, Wu X, et al. Nitrous oxide plus isoflurane induces apoptosis and increases beta-amyloid protein levels. Anesthesiology 2009;111:741-52. [Crossref] [PubMed]
- Zhang Y, Zhen Y, Dong Y, et al. Anesthetic propofol attenuates the isofluraneinduced caspase-3 activation and Aβ oligomerization. PLoS One 2011;6:e27019 [Crossref] [PubMed]
- Fodale V, Santamaria L, Schifilliti D, et al. Anaesthetics and postoperative cognitive dysfunction: a pathological mechanism mimicking Alzheimer’s disease. Anaesthesia 2010;65:388-95. [Crossref] [PubMed]
- Run X, Liang Z, Gong C. Anesthetics and tau protein: animal model studies. J Alzheimers Dis 2010;22:49-55. [Crossref] [PubMed]
- Lu H, Liufu N, Dong Y, et al. Sevoflurane acts on ubiquitination-proteasome pathway to reduce postsynaptic density 95 protein levels in young mice. Anesthesiology 2017;127:961-75. [Crossref] [PubMed]
- Xu G, Lu H, Dong Y, et al. Coenzyme Q10 reduces sevoflurane-induced cognitive deficiency in young mice. Br J Anaesth 2017;119:481-91. [Crossref] [PubMed]
- Hou JF, Xiao CL. Effect of propofol and sevoflurane anesthesia on postoperative cognitive function and levels of Aβ-42 and Tau in patients undergoing hepatectomy. Eur Rev Med Pharmacol Sci 2019;23:849-56. [PubMed]
- Barmack NH, Yakhnitsa V. Climbing fibers mediate vestibular modulation of both ‘complex’ and ‘simple spikes’ in Purkinje cells. Cerebellum 2015;14:597-612. [Crossref] [PubMed]
- Huang JJ, Yen CT, Tsao HW, et al. Neuronal oscillations in Golgi cells and Purkinje cells are accompanied by decreases in Shannon information entropy. Cerebellum 2014;13:97-108. [Crossref] [PubMed]
- Jacobs HIL, Hopkins DA, Mayrhofer HC, et al. The cerebellum in Alzheimer's disease: evaluating its role in cognitive decline. Brain 2018;141:37-47. [Crossref] [PubMed]
- Mavroudis IA, Fotiou DF, Adipepe LF, et al. Morphological changes of the human purkinje cells and deposition of neuritic plaques and neurofibrillary tangles on the cerebellar cortex of Alzheimer's disease. Am J Alzheimers Dis Other Demen 2010;25:585-91. [Crossref] [PubMed]
- Sepulveda-Falla D, Barrera O, Hagel C, et al. Familial Alzheimer’s disease-associated presenilin-1 alters cerebellar activity and calcium homeostasis. J Clin Invest 2014;124:1552-67. [Crossref] [PubMed]
- Irie T, Kikura H, Usami M, et al. MAM-2201, a synthetic cannabinoid drug of abuse, suppresses the synaptic input to cerebellar Purkinje cells via activation of presynaptic CB1 receptor. Neuropharmacology 2015;95:479-91. [Crossref] [PubMed]
- Irifune M, Takarada T, Shimizu Y, et al. Propofol-induced anesthesia in mice is mediated by γ-aminobutyric acid-A and excitatory amino acid receptors. Anesth Analg 2003;97:424-9. [Crossref] [PubMed]
- Zhang XY, Zhang YD, Cui BR, et al. Propofol facilitates climbing fiber-Purkinje cell synaptic transmission via NMDA receptor in vitro in mice. Eur J Pharmacol 2020;887:173474 [Crossref] [PubMed]
- Brooks DE. Propofol-induced movement disorders. Ann Emerg Med 2008;51:111-2. [Crossref] [PubMed]
- Jin R, Liu H, Jin W, et al. Propofol depresses cerebellar Purkinje cell activity via activation of GABA(A) and glycine receptors in vivo in mice. Eur J Pharmacol 2015;764:87-93. [Crossref] [PubMed]
- Bellamy TC. Interactions between Purkinje neurones and Bergmann glia. Cerebellum 2006;5:116-26. [Crossref] [PubMed]
- Lütolf S, Radtke F, Aguet M, et al. Notch1 is required for neuronal and glial differentiation in the cerebellum. Development 2002;129:373-85. [Crossref] [PubMed]
- Komine O, Nagaoka M, Watase K, et al. The monolayer formation of Bergmann glial cells is regulated by Notch/RBP-J signaling. Dev Biol 2007;311:238-50. [Crossref] [PubMed]
- Fang H, Wang Z, Bu Y, et al. Repeated inhalation of sevoflurane inhibits the information transmission of Purkinje cells and delays motor development via the GABAA receptor ε subunit in neonatal mice. Mol Med Rep 2018;17:1083-92. [PubMed]
- Carnini A, Lear J, Eckenhoff R. Inhaled anesthetic modulation of amyloid beta(1-40) assembly and growth. Curr Alzheimer Res 2007;4:233-41. [Crossref] [PubMed]
- Song B, Li Y, Teng X, et al. Comparison of Morning and Evening Operation Under General Anesthesia on Intraoperative Anesthetic Requirement, Postoperative Sleep Quality, and Pain: A Randomized Controlled Trial. Nat Sci Sleep 2020;12:467-75. [Crossref] [PubMed]
- Lazic K, Petrovic J, Ciric J, et al. REM sleep disorder following general anesthesia in rats. Physiol Behav 2017;168:41-54. [Crossref] [PubMed]
- Kain ZN, Caldwell-Andrews AA. Sleeping characteristics of adults undergoing outpatient elective surgery: a cohort study. J Clin Anesth 2003;15:505-9. [Crossref] [PubMed]
- Palma JA, Urrestarazu E, Iriarte J. Sleep loss as risk factor for neurologic disorders: a review. Sleep Med 2013;14:229-36. [Crossref] [PubMed]
- Westermann J, Lange T, Textor J, et al. System consolidation during sleep—a common principle underlying psychological and immunological memory formation. Trends Neurosci 2015;38:585-97. [Crossref] [PubMed]
- Mander BA, Winer JR, Jagust WJ, et al. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci 2016;39:552-66. [Crossref] [PubMed]
- Irwin MR, Opp MR. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology 2017;42:129-55. [Crossref] [PubMed]
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 2015;16:358-72. [Crossref] [PubMed]
- Bero AW, Yan P, Roh JH, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci 2011;14:750-6. [Crossref] [PubMed]
- Irwin MR. Why sleep Is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol 2015;66:143-72. [Crossref] [PubMed]
- Wood H. Dementia: Peripheral inflammation could be a prodromal indicator of dementia. Nat Rev Neurol 2018;14:127. [Crossref] [PubMed]
- King E, O’Brien JT, Donaghy P, et al. Peripheral inflammation in prodromal Alzheimer’s and Lewy body dementias. J Neurol Neurosurg Psychiatry 2018;89:339-45. [Crossref] [PubMed]
- van der Kant R, Goldstein L. Cellular functions of the amyloid precursor protein from development to dementia. Dev Cell 2015;32:502-15. [Crossref] [PubMed]
- Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673-734. [Crossref] [PubMed]


