
Analysis on the value of soluble intercellular adhesion molecule-1 (sICAM-1), alpha fetoprotein (AFP), and aspartate aminotransferase/platelet ratio index (APRI) in predicting the prognostic survival of patients with primary liver cancer after radiofrequency ablation
Introduction
Hepatocellular carcinoma (HCC) is one of the more common malignant tumors in China, with the incidence of this disease increasing in recent years (1). With the advancement of various treatment methods, the current clinical treatment of HCCis mainly radiofrequency ablation (RFA), but long-term clinical applications have found that this treatment has low efficiency and yields a short median survival time (2). Therefore, identifying the factors that affect the RFA treatment of HCC patients is crucial for developing timely and effective intervention measures (3).
With the development of cell and biomolecular research, the molecular mechanism for the onset and metastasis of HCC patients and new targets for tumor therapy have attracted widespread attention (4). Soluble intercellular adhesion molecule-1 (sICAM-1) is a member of the immunoglobulin family, and plays an important role in the process of antigen-specific cytotoxicity, immune complex formation, and target cell lysis (5). Previous research have showed that Serum sICAM-1 may be a promising predictor for the overall and recurrence-free survival of patients with HCC (6). At the same time, the role of proteasome, sICAM-1, sTNFR-II and β-catenin in early detection of HCC. Also, using this panel of serological markers in combination with αFP may offer improved diagnostic performance over αFP alone in the early detection of HCC (7). Alpha fetoprotein (AFP) is an important indicator for the specific diagnosis of liver cancer, which has important value in the diagnosis of early liver cancer patients and the screening of high-risk populations (8). In addition, related studies have found that aspartate aminotransferase/platelet ratio index (AST/PLT ratio index, APRI) is closely related to liver function and can affect the prognosis of patients with liver cancer after surgery (9). Therefore, we enrolled 115 HCC patients in a research group, and analyzed the expression of sICAM-1, AFP, and APRI, and their relationship with prognostic survival after RFA treatment in HCC patients. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/apm-21-749).
Methods
General information
We retrospectively analyzed 115 HCC patients admitted to our hospital from June 2016 to June 2018. There were 59 male patients and 56 female patients, aged from 31 to 75 years old, with an average age of 60.31±6.65 years old. All patients agreed to participate in this study and signed an informed consent form. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by The First Affiliated Hospital of Zhengzhou University Academy of Medical Sciences of Zhengzhou University (No. 20160522). The inclusion criteria were as follows: (I) confirmed by pathological examination, (II) complete clinical data, and (III) no other malignant diseases. The exclusion criteria were as follows: (I) severe diseases of the kidneys and heart organs, (II) severe neurological or cognitive dysfunction, and (III) with poor compliance.
Another 120 healthy people who were examined during the same period were selected as the control group. This group consisted of 63 males and 57 females, aged from 32 to 76 years old, with an average of 60.69±6.75 years old. There was no difference in general characteristics, such as gender and age, between the study group and the control group.
RFA treatment
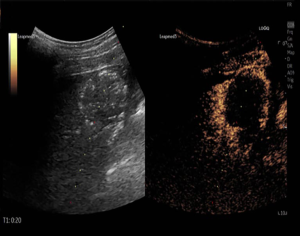
For RFA treatment (10), the radio frequency instrument used was the Cool-Tip instrument (Radionics). Ultrasound detection was used to locate and mark the cancer. The patient was given 0.5% lidocaine for local anesthesia plus intravenous general anesthesia. The radiofrequency treatment needle was inserted along the long diameter of the tumor to the bottom of the lesion, and the cold circulation was connected. The impedance mode, cold circulation electrode needle was used to start radiofrequency treatment. The current power was 85–130 mA, the impedance was 80–140 Ω, and the frequency was 90–120 Hz. Cooling water in a circulating pump was used to maintain the temperature of the electrode tip at about 28 °C. Under the guidance of ultrasound, the radiofrequency electrode needle was ablated at the lesion 3 to 4 times, with an interval of 1 to 2 minutes each time. Ultrasound was used to detect the return light mass during the treatment. The number of needle withdrawals was determined according to the size of the tumor and the scope of damage to the normal liver tissue (0.5 to 1.0 cm outside the tumor edge). The video stills of RFA treatment for primary liver cancer are shown in Figures 1, 2, and 3.

Specimen collection and testing methods
In the morning, 5 mL of blood was collected from all patients on an empty stomach with a standard sampling tube without anticoagulant. After centrifugation (3,000 r/min, 10 min), the serum was collected and stored in a polypropylene Eppendorf (EP) tube, and kept in a –80 °C freezer. Enzyme-linked immunosorbent assay (ELISA) was used to detect sICAM-1. The electrochemiluminescence method was used to detect AFP. A Roche C800 automatic biochemical analyzer was used to detect AST and alanine aminotransferase (ALT), and the APRI value was calculated (AST/PLT ratio index). All operations were performed in accordance with the kit manufacturer’s instructions.
Follow-up
The follow-up period was from the patient’s treatment to 2 years after treatment, ending in June 2020. The follow-up time of patients was 2–24 months, and the average follow-up time was (15.71±5.93) months. Follow-up of patients was mainly completed through telephone, We Chat, and patients’ visit to the hospital for review.
Statistical methods
The data in this study were statistically analyzed using SPSS22.0 software (IBM Corp). The count data are expressed as numbers and percentages, and were analyzed using the t test; meanwhile, measurement data are expressed as mean ± standard deviation (SD), and were analyzed with the χ2 test. Multivariate logistic regression analysis was used to analyze the risk factors affecting the prognosis and survival of HCC patients. Receiver operating characteristic (ROC) was used to analyze the predictive value of sICAM-1, AFP, and APRI in the survival of patients with advanced HCC after RFA treatment. A P value <0.05 was considered to indicate a statistically significant difference.
Results
Levels of sICAM-1, AFP, and APRI between the two groups
As shown in Table 1, the levels of sICAM-1, AFP, and APRI in the control group were significantly lower than those in the study group (P<0.05).

Full table
The prognosis of HCC patients after RFA treatment
After 2 years of follow-up, the 2-year survival rate was 55.65% (64/115).
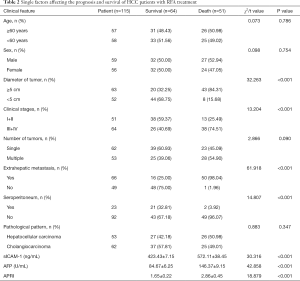
Single factors affecting the prognosis and survival of HCC patients with RFA treatment
As shown in Table 2, there was no difference in the survival rate of patients with different ages, gender, tumor numbers, or pathological types; meanwhile, the survival rates did vary significantly according to clinical stage, tumor diameter, extrahepatic metastasis, abdominal effusion, and sICAM-1, AFP, and APRI levels (all P values<0.05).
Full table
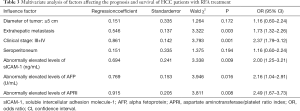
Multivariate analysis of factors affecting the prognosis and survival of HCC patients with RFA treatment
As shown in Table 3, Clinical stage (III + IV), extrahepatic metastasis, and abnormally elevated levels of sICAM-1, AFP, and APRI were independent risk factors that affected the prognosis and survival of HCC patients after RFA treatment (P<0.05).
Full table
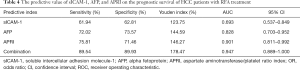
The predictive value of sICAM-1, AFP, and APRI on the prognostic survival of HCC patients with RFA treatment
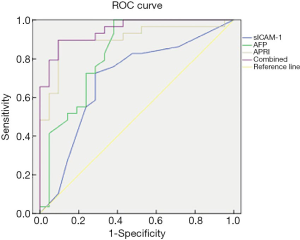
13 An ROC curve was used to analyze the predictive value of sICAM-1, AFP, APRI and their combination for survival of HCC patients after RFA treatment (Table 4 and Figure 4). The area under the curve (AUC) of sICAM-1, AFP, APRI, and their combination was 0.693, 0.828, 0.901, and 0.947, respectively. As the AUC of the combined indicator was the largest, this indicator showed the best predictive ability.
Full table
Discussion
HCC is a malignant tumor with global reach and high mortality. The pathogenesis of the disease is unclear, and there are no obvious symptoms in the early stage. In the later stage, patients may experience symptoms of ascites, jaundice, and cachexia, which threaten the patient’s life and safety (11,12). RFA represents a breakthrough in the field of HCC treatment in recent years, but the treatment effect in some patients is still not ideal, resulting in poor prognosis (13). Therefore, finding effective therapeutic targets is crucial for improving the prognosis of HCC patients with RFA (14).
sICAM-1 is a member of the family of intercellular adhesion molecules and exists on cell surface glycoproteins. It can stimulate the adhesion between cells and extracellular matrix, and participates in the maintenance of normal tissue structure, embryonic differentiation and development, tumor infiltration, and immune regulation (15,16). Related studies have found that sICAM-1 produced by the shedding of tumor cells can effectively block the adhesion of toxic T lymphocytes and natural killer (NK) cells, and destroy tumor cells (17). At present, studies have confirmed that sICAM-1 is closely related to the progression of liver cancer, and its level and concentration are positively expressed in liver cancer tissues (18). In addition, researchers have used different treatment methods to detect the levels of sICAM-1 in HCC patients. They have found that the levels of sICAM-1 are decreased most significantly in patients undergoing surgery following interventional therapy and that this change is closely related to extrahepatic metastasis, tumor staging, and other processes (19). This is in line with the results of our study, suggesting that the levels of sICAM-1 can reflect the malignant degree of liver tumors and the ability of liver cancer cells to invade and metastasize to a certain extent. Thus, sICAM-1 may be an effective indicator of tumor development, metastasis, and prognosis.
APRI is a widely used clinical, noninvasive, diagnostic index for liver cirrhosis, and has gradually become a focus of liver cancer-related research. AST is an important transaminase in the body, being widely present in various tissues and acting as an effective indicator of liver cell necrosis (20). A large number of studies have shown that platelets play multiple roles in the process of inflammation, and can promote cancer progression and metastasis by promoting angiogenesis and the production of adhesion molecules (21). Related studies have indicated that APRI can be used as an indicator of the prognosis of patients with liver cancer after surgery and is an independent factor that affects the overall survival of patients after surgery (22). Studies have shown that after RFA treatment is applied in HCC patients, liver cancer cells continue to deteriorate without being inhibited, which aggravates liver cell damage, resulting in increased levels of AST and ALT, and the proliferation of tumor neovascularization (23). Our study found that the level of APRI was significantly increased in tumor tissues, and its high expression was related to the prognostic survival rate of patients with RFA.
AFP is an acid glycoprotein. In recent years, it has been discovered that AFP can be produced by malignant tumors of the reproductive organs. Jonas et al. (24) found that during the progression of HCC disease, the excessive proliferation of cancer cells could promote the rapid proliferation and division of cancer cells; furthermore, AFP could be rapidly activated through special channels and released into the blood, and thus be highly expressed. Relevant studies have shown that AFP levels can not only reflect tumor cell proliferation and migration, but also reflect potential liver necrosis and regeneration, and can be used as an important indicator for evaluating the efficacy of treatment and determining HCC prognosis (25). Our study demonstrated that the level of AFP was closely related to the prognosis and survival of patients after RFA treatment, indicating that it may be used in an auxiliary role for evaluating the occurrence and development of HCC and subsequent therapeutic effects. However, it is worth noting that due to the diversity and complexity of the biological characteristics of malignant tumors, some indicators may show different expressions in different HCC patients. Therefore, the sensitivity sICAM-1, AFP, and APRI detection alone in the diagnosis of HCC was not high. In order to improve the effectiveness of clinical diagnosis and prognosis evaluation of HCC, an ROC curve was employed. This showed that the combination of sICAM-1, AFP, and APRI levels had the best predictive value for the prognosis of patients with RFA treatment, indicating that the combined indicators could be used as an effective means to predict the prognosis of patients with RFA treatment.
In summary, sICAM-1, AFP, and APRI are closely related to the RFA treatment and the prognosis of HCC patients. Clinically, individualized treatment plans can be made based on these level changes and may contribute to prolonging the survival of patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-749
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-749
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-749). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients agreed to participate in this study and signed an informed consent form. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by The First Affiliated Hospital of Zhengzhou University Academy of Medical Sciences of Zhengzhou University (No. 20160522).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chaudhari VA, Khobragade K, Bhandare M, et al. Management of fibrolamellar hepatocellular carcinoma. Chin Clin Oncol 2018;7:51. [Crossref] [PubMed]
- Zhang X, Li J, Shen F. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 2018;33:347-54. [Crossref] [PubMed]
- Papageorgiou C, Jourdi G, Adjambri E, et al. Disseminated Intravascular Coagulation: An Update on Pathogenesis, Diagnosis, and Therapeutic Strategies. Clin Appl Thromb Hemost 2018;24:8s-28s. [Crossref] [PubMed]
- Ueno M, Takabatake H, Kayahara T, et al. Mucin-producing hepatocellular carcinoma without morphological features of biliary differentiation: A case report. Medicine (Baltimore) 2018;97:e12159 [Crossref] [PubMed]
- Tasse JC, Sailer A, Jakate S, et al. Transportal Chemoembolization as Salvage Hepatocellular Carcinoma Therapy to Maintain Liver Transplant Candidacy. J Vasc Interv Radiol 2019;30:1651-4.e1. [Crossref] [PubMed]
- Shimura T, Shibata M, Gonda K, et al. Prognostic impact of soluble intercellular adhesion molecule-1 in hepatocellular carcinoma. Oncol Lett 2018;16:6013-8. [Crossref] [PubMed]
- Zekri AR, Youssef AS, Bakr YM, et al. Serum biomarkers for early detection of hepatocellular carcinoma associated with HCV infection in egyptian patients. Asian Pac J Cancer Prev 2015;16:1281-7. [Crossref] [PubMed]
- Salati U, Barry A, Chou FY, et al. State of the ablation nation: a review of ablative therapies for cure in the treatment of hepatocellular carcinoma. Future Oncol 2017;13:1437-48. [Crossref] [PubMed]
- Wang YM, Lyon J, Yoon JM. Transcatheter arterial embolization as palliation in metastatic hepatocellular carcinoma. Pediatr Blood Cancer 2019;66:e27754 [Crossref] [PubMed]
- Park SI, Kim IJ, Lee SJ, et al. Angled Cool-Tip Electrode for Radiofrequency Ablation of Small Superficial Subcapsular Tumors in the Liver: A Feasibility Study. Korean J Radiol 2016;17:742-9. [Crossref] [PubMed]
- Pearson H, Knisely AS, Deheragoda M, et al. Treatment of metastatic hepatocellular carcinoma in pediatric patients: Two case reports. Pediatr Hematol Oncol 2018;35:90-4. [Crossref] [PubMed]
- Eatrides J, Wang E, Kothari N, et al. Role of Systemic Therapy and Future Directions for Hepatocellular Carcinoma. Cancer Control 2017;24:1073274817729243 [Crossref] [PubMed]
- Nishida N. Long-term prognosis and management of hepatocellular carcinoma after curative treatment. Clin Mol Hepatol 2020;26:480-3. [Crossref] [PubMed]
- Schlabritz-Loutsevitch N, Carrillo M, Li C, et al. A first case of hepatocellular carcinoma in the baboon (Papio spp.) placenta. J Med Primatol 2019;48:68-73. [Crossref] [PubMed]
- Shiani A, Narayanan S, Pena L, et al. The Role of Diagnosis and Treatment of Underlying Liver Disease for the Prognosis of Primary Liver Cancer. Cancer Control 2017;24:1073274817729240 [Crossref] [PubMed]
- Usmani A, Mishra A, Ahmad M. Nanomedicines: a theranostic approach for hepatocellular carcinoma. Artif Cells Nanomed Biotechnol 2018;46:680-90. [Crossref] [PubMed]
- Sun F, Wang JZ, Luo JJ, et al. Exosomes in the Oncobiology, Diagnosis, and Therapy of Hepatic Carcinoma: A New Player of an Old Game. Biomed Res Int 2018;2018:2747461 [Crossref] [PubMed]
- Goh BK, Chung AY. Response to LTE regarding-Importance of tumor size as a prognostic factor after partial liver resection for solitary hepatocellular carcinoma: Implications on the current AJCC staging system. J Surg Oncol 2016;113:594. [Crossref] [PubMed]
- Denlinger C. Therapy Options for Patients With Hepatocellular Carcinoma. Oncology (Williston Park) 2019;33:685018 [PubMed]
- Weeda VB, Aronson DC. Is hepatocellular carcinoma the same disease in children and adults? Comparison of histology, molecular background, and treatment in pediatric and adult patients. Pediatr Blood Cancer 2019;66:e27475 [Crossref] [PubMed]
- Akkız H. Hepatocellular Carcinoma: From Molecular Basis to Novel Treatment Approaches. Can J Gastroenterol Hepatol 2019;2019:4970731 [Crossref] [PubMed]
- Liu Y, Sun L, Gao F, et al. A new scoring model predicting macroscopic vascular invasion of early-intermediate hepatocellular carcinoma. Medicine (Baltimore) 2018;97:e13536 [Crossref] [PubMed]
- Titano J, Voutsinas N, Kim E. The Role of Radioembolization in Bridging and Downstaging Hepatocellular Carcinoma to Curative Therapy. Semin Nucl Med 2019;49:189-96. [Crossref] [PubMed]
- Jonas E. Hepatocellular carcinoma in sub-Saharan Africa - the way forward. S Afr Med J 2018;108:12391. [PubMed]
- Cho E, Cho HA, Jun CH, et al. A Review of Hepatocellular Carcinoma in Elderly Patients Focused on Management and Outcomes. In Vivo 2019;33:1411-20. [Crossref] [PubMed]
(English Language Editor: J. Gray)



