
Factors associated with bone metastasis in breast cancer: a systematic review and meta-analysis
Introduction
Breast cancer (BC) is one of the most common malignant tumors in women, and its incidence rate has increased in recent years. Bone tissue is the most common metastatic site of advanced BC, and the proportion of metastasis in the bone is much higher than the proportions of metastases in other organs. A meta-analysis in 2017 showed that the 5-year incidence of bone metastasis (BM) in patients with stages I–III BC was 12% (1). A SEER based study found that BM of BC patients with distant metastasis was 51% (8,848/17,445), which is higher than lung metastasis 24% (4,167/17,445), liver metastasis 20% (3,434/17,445) and brain metastasis 6% (1,000/17,445) (2). Leone et al. reported that BM rate was 37.5%, visceral metastasis rate was 21%, and other site metastasis rate was 11.9% in 9,143 patients of IV BC at initial diagnosis (3).
BM of BC can induce anemia, fractures, paraplegia, pain, cachexia, and other conditions, and significantly reduces the quality of life of patients. Factors related to BM in BC patients can be used as screening tools for the prevention and treatment of BM, and are very important for reducing adverse events associated with BM and improving the quality of life of patients. BM of BC can occur in the ribs, vertebra, femur, ilium, and other bones. The process of the development of secondary bone tumors after tumor metastasis to the bone through the lymphatic system and blood circulation is extremely complicated, and the mechanism is still not completely clear. From the “seeds and soil” theory proposed 100 years ago to the “homing” theory suggested today, all theories support the specific rather than random metastasis of cancer cells.
Currently, the meta-analysis is mainly focuses on the treatment of BC patients with BM. Awan et al. analysed 5 clinical trials, and the results showed Zoledronate administration every 12 weeks compared to every 4 weeks for patients with 1 on-study SRE indicating similar efficacy (4). Yang et al. analyzed 4 articles to compare the efficacy of bisphosphonate treatment every 4 weeks to every 12 weeks, and there were no significant differences in skeletal-related events, renal dysfunction, and osteonecrosis of jaw (5).
With the continuous updating of clinical indicators, some new clinical indicators have been incorporated into the evaluation of BM, such as hormone receptors (HRs) and BC staging. The addition of these new indicators changes the traditional model, which had fewer indicators, such as tumor size and TNM stage, and improves the accuracy of the prediction of BC BM. Zhang et al. systematically extrapolated the occurrence, risk factor, prognostic characteristics, BM and SREs, but they did not calculate the odds ratio (OR) of each index (6). In this report, retrospective studies on the BM of BC were reviewed, and a meta-analysis was performed to evaluate the predictive indexes.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-21-438).
Methods
Retrieval strategy studies were retrieved from the Cochrane Library, PubMed, Web of Science, and EMBASE. Included articles were published between January 1, 2001, and December 31, 2019
The Cochrane Library search strategies (((Breast Neoplasms)OR(Breast Neoplasm)OR(Breast cancer)OR(Breast Carcinoma)OR(Breast Tumor))AND(Bone metastasis)OR(metastasis of Bone)OR((Skeletal metastasis)OR(Skeletal complication)OR(skeletal-related event))AND(Publication Date from January 1st,2001 to December 31,2020)).
PubMed and EMBASE search strategies (((Breast Neoplasms[Mesh])OR(Breast Neoplasm)OR(Breast cancer)OR(Breast Carcinoma)OR(Breast Tumor))AND(Bone metastasis)OR(metastasis of Bone)OR((Skeletal metastasis)OR(Skeletal complication)OR(skeletal-related event)) AND(limits:Humans,English;Publication Date from January 1st,2001 to December 31,2020)).
Web of Science search strategies (TS=((Breast Neoplasms[Mesh])OR(Breast Neoplasm)OR(Breast cancer)OR(Breast Carcinoma)OR(Breast Tumor)) AND(TS=((Bone metastasis)OR(metastasis of Bone)OR(Skeletal metastasis)OR(Skeletal complication)OR(skeletal-related event))) AND (DT=Article),AND (English,PY=(2001-2020)).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) the study subjects were diagnosed with BC, and patients with bone metastases were diagnosed by imaging or pathology; (II) complete data could be extracted, and the hazard ratio (HR) and 95% confidence interval (CI) could be directly or indirectly obtained; (III) more than 1 index was included; and (IV) the number of patients with bone metastases from BC was not fewer than 10.
The exclusion criteria were as follows: (I) duplicates, reviews, and case reports or studies for which the full text versions were unavailable; (II) the included patients had other tumors at the same time; (III) the data could not be extracted or were incomplete; and (IV) studies with a Newcastle-Ottawa scale (NOS) score ≤5.
The indicators of interest were as follows: (I) estrogen receptor (ER) expression; (II) progesterone receptor (PR) expression; (III) HR expression, with positive ER or PR expression indicating positive HR expression; (IV) human epidermal growth factor receptor-2 (HER2) expression; (V) luminal A, luminal B, HER2 overexpression, and triple-negative breast cancer (TNBC) subtypes; (VI) premenopausal and postmenopausal status; (VII) histological grades I, II, and III; (VIII) positive or negative lymph node metastasis status, where negative status indicated that the number of lymph node metastases was 0 or the N stage was N0, and positive status indicated that the number of lymph node metastases was ≥1 or the N stage was N1–N4; (IX) tumor size; (X) TNM T stage (T1, T2, T3, T4); and (XI) histological types determined by pathology, including ductal, lobular, and others (mixed ductal and lobular, mucinous, papillary, carcinoma)
Study screening and evaluation
Literature screening and evaluation were performed independently by 2 evaluators. First, irrelevant documents were excluded by reading the title and abstract. Then the full texts of the remaining documents were read, and the final selection was made based on the inclusion and exclusion criteria. The quality of the included studies was evaluated with the NOS as recommended by the Cochrane Collaboration Network (cohort study) (7). The maximum score was 9, and studies with scores ≥6 were high-quality studies, while those with scores ≤5 were low-quality studies. In cases of disputes, the evaluations were negotiated or assessed by an experienced third party.
Data extraction
The extracted data were the title, year of publication, author, study design, and observation index. The statistics of indicators were extracted directly from the text when available; otherwise, they were calculated indirectly. The data entry was performed by 2 evaluators independently. If the data entered was inconsistent, the data was discussed and corrected through negotiation or adjudication by an experienced third party. If there were subjects from the same region (database) in the same period in more than 1 study, only data from 1 of the studies was included for each indicator of interest.
Statistical methods
The original data was entered into Excel 2006. Data conversion and statistical analyses of the indicators of interest were performed with Stata 12.0 statistical software. The weighted mean difference (WMD) was used for measurement data, and the OR was used for count data. If the original data were HRs and 95% CI, then the HR was considered to be equivalent to the OR. All effect indexes are presented with the corresponding 95% CIs. The natural logarithm of the OR (lnOR) and the natural logarithm of the 95% CI (lnLL, lnUL) were calculated. Then, the natural logarithm of the standard error was calculated with the following equation: SElnOR = ln(lnUL-lnLL)/3.92. Heterogeneity was tested using the chi-square test. If I2 was <50%, the heterogeneity was acceptable, and the fixed effect model was used for the analysis. If I2 was greater than 50%, the level of heterogeneity was high, and a random effect model was used for the analysis. Publication bias was tested by the Begg’s rank correlation method. P<0.05 was statistically significant.
Results
Results of the literature search
The searches of PubMed, Web of Science, EMBASE, and the Cochrane Library yielded 3,157 articles. Batch and manual checks for duplicates were performed using EndNote software. A total of 1,921 documents remained after eliminating duplicate documents, and 1,625 irrelevant documents were excluded after reading the title and abstract. Of the 69 documents that remained, 51 were removed after reading the full texts and assessing the study quality. Finally, 18 documents were included in the meta-analysis (Figure 1).
Basic information (Table 1)
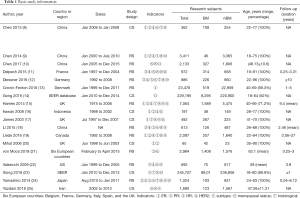
Full table
Eighteen studies (8-25) were included from 13 countries, including China, France, Germany, Denmark, the UK, Italy, Indonesia, Canada, the US, Belgium, Spain, Iran, and Japan. An additional 6 European countries (Belgium, France, Germany, Italy, Spain, and the UK) were included in the study by Von Moos et al. in 2018 (21) and other studies. Gong et al. in 2018 collected 229,195 subjects from the Surveillance, Epidemiology, and End Results (SEER) database between January 2010 and December 2014 (14). Xiong et al. in 2018 collected 245,707 subjects from the SEER database between January 2010 and December 2013 (23). There was obvious overlap between the 2 research datasets. This study mainly used the data from the study by Xiong et al. in 2018 (23). Data on subtypes, histological grades, nodal metastasis, T stages, and other indicators of interest were collected. The use of the histological type obtained from pathological reports in the study by Gong et al. in 2018 (14) was intended to maximize the data and prevent the inclusion of duplicate data in the meta-analysis. Eleven retrospective studies and 6 cross-sectional studies were included.
Quality assessment
The NOS scores ranged from 6–9, including 7 studies scoring 6 points, 5 studies scoring 7 points, 4 studies scoring 8 points, and 2 studies scoring 9 points (Table 2).
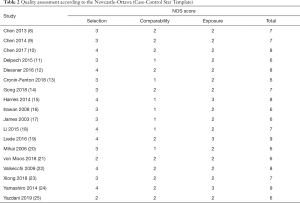
Full table
Meta-analysis overview
Eleven indicators were included in this study. Tumor size was only preliminarily analyzed because of a lack of standard definitions in the literature. Among the other 10 indicators, subgroup analyses were performed for the subtype, histological grade, and T stage, etc. Nodal metastasis was defined as the presence of metastatic lymph nodes and was further grouped based on the cutoff value of ≥3 involved lymph nodes. The I2 for PR was 45.9%, and a fixed effect model was used. The I2 values for the other 9 indicators were all >50%, and random effects models were used. Begg’s test showed that there was no statistically significant bias caused by any study, and the meta-analysis was performed by pooling the data (Table 3).
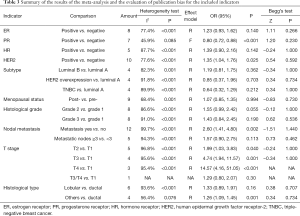
Full table
Analysis results
ER
Eight studies (8,9,13,15-17,19,20) reported the effect of ER status on BM of BC. No significant differences were observed in the incidence of BM between ER-positive and ER-negative BC patients (I2=77.4%, OR =1.23, 95% CI: 0.93, 1.62, P=0.140) (Figure 2).
PR
Seven studies (8,9,15,16,19,20,24) reported the effect of PR status on BM of BC. The results showed that the risk of BM was lower in PR-positive than in PR-negative BC patients (I2=45.9%, OR =0.80, 95% CI: 0.72, 0.88, P<0.001) (Figure 3).
HR
Five studies (11,12,18,21,22) analyzed the effect of HR status on BM of BC. The results showed that the risk of BM was higher in HR-positive than in HR-negative BC patients (I2=87.7%, OR =1.39, 95% CI: 0.90, 2.16, P=0.142) (Figure 4).
HER2
Ten studies (8,9,11,12,15,16,18,19,22,24) evaluated the risk of HER2 on BM from BC. The results showed that HER2-positive BC patients had a relatively higher risk of BM (I2=77.6%, OR =1.35, 95% CI: 1.04, 1.76, P=0.025) (Figure 5).
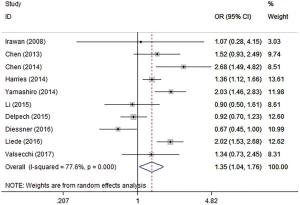
Subtype
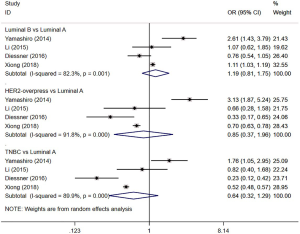
Four studies (12,18,23,24) evaluated the risk of BM in patients with different subtypes of BC. The results showed that the incidence of BM in patients with luminal A BC was not significantly different from that in patients with the following subtypes: luminal B (I2=82.3%, OR =1.19, 95% CI: 0.81, 1.75, P=0.362), HER2 overexpression (I2=91.8%, OR =0.85, 95% CI: 0.37, 1.96, P=0.703), and TNBC (I2=89.9%, OR =0.64, 95% CI: 0.32, 1.29, P=0.212) (Figure 6).
Menopausal status
Nine studies (8,9,11,12,18,21,22,24,25) reported the effect of menopause on BM in BC patients. The results showed that the risk of BM in postmenopausal BC patients was not significantly different from that in premenopausal patients (I2=68.4%, OR =1.07, 95% CI: 0.85, 1.35, P=0.994) (Figure 7).
Histological grade
Eight studies (8,9,12,15,17,19,23,24) reported the risk of BM in patients with different histological grades of BC. No significant difference was observed in the incidence of BM between patients with grade 2 (I2=86.6%, OR =1.55, 95% CI: 0.99, 2.42, P=0.055) or grade 3 BC (I2=91.0%, OR =1.43, 95% CI: 0.84, 2.45, P=0.190) and those with grade 1 BC (Figure 8).
Nodal metastasis
Twelve studies (8-12,15,17-19,23-25) compared the effect of nodal metastasis on the BM of BC. Chen et al. [2014] (9), Xiong et al. [2018] (23), Yamashiro et al. [2014] (24), and Yazdani et al. [2019] (25) reported the N stage. The results showed that the risk of BM in patients with lymph node metastasis was higher than that in patients with no lymph node metastasis, and the difference was statistically significant (I2=99.7%, OR =2.60, 95% CI: 1.41, 4.80, P=0.002) (Figure 9).
Five studies (8,10,12,15,17) evaluated the effect of the number of metastatic lymph nodes on the BM of BC. The results showed that there was no significant difference between patients with ≥3 and <3 metastatic lymph nodes (I2=94.3%, OR =1.57, 95% CI: 0.90, 2.75, P=0.113) (Figure 10).
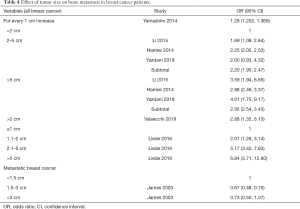
Tumor size
Seven studies (15,17-19,22,24,25) reported the effect of tumor size on BM in BC patients (Table 4). The subjects in 6 studies (15,18,19,22,24,25) were all stage I–IV BC patients. The results suggest that the larger the tumor is, the higher the risk of BM. Since the parameters used to stratify the tumor sizes in each study were different, the data were not combined. James et al. showed that BC patients with tumor metastasis with tumors ≥1.5 cm had a lower probability of BM than patients with tumors <1.5 cm (17).
Full table
T stage
Five studies (1,9,12,23,24) reported the risk of BM in BC patients with different T stages (Figure 11). The results showed that the higher the T stage, the higher the risk of BM. Data from 5 studies (7,9,11,23,24) showed that the risk of BM in stage T2 BC patients was 1.99 times that of patients with stage T1 BC (I2=96.8%, OR =1.99, 95% CI: 1.03, 3.83, P=0.040). The data from 4 studies (9,11,23,24) showed that the risk of BM of stage T3 BC patients was 4.74 times that of stage T1 BC patients (I2=95.6%, OR =4.74, 95% CI: 1.94, 11.57, P=0.001). The combined data of 3 studies (9,23,24) showed that the risk of BM in stage T4 BC patients was 14.57 times that of stage T1 BC patients (I2=95.4%, OR =14.57, 95% CI: 4.16, 51.05, P<0.001). One (12) study reported that the BM rate of stage T3/T4 BC patients was not significantly different from that of stage T1 BC patients (OR =1.29, 95% CI: 0.80, 2.07, P=0.30).
Histological type
Six studies (10,11,14-17) compared the risk of BM in patients with BC of different histological types (Figure 12). The meta-analysis results of 6 studies (10,11,14-17) showed that the incidence of BM in lobular BC patients was not significantly different from that in ductal BC patients (I2=93.6%, OR =1.33, 95% CI: 0.89, 1.97, P=0.16). Four studies (11,14,15,17) showed that the incidence of BM in non-lobular and non-ductal BC patients was higher than that in patients with ductal or lobular BC, and the difference was statistically significant (I2=56.4%, OR =1.26, 95% CI: 1.09, 1.45, P=0.001).
Sensitivity analysis
In this study, the heterogeneity of PR was small (I2=45.9%, P=0.085), and the heterogeneity of other indicators was large (I2>50%, P<0.05). After the sensitivity analysis using the elimination method, the I2 value and the scale of combined effect did not change significantly, indicating that the research results are robust.
Publication bias
In this paper, we conducted Begg’s test on the indexes with more than 4 included studies, and the results showed no obvious publication bias (Table 3).
Discussion
HR
The breast is a target organ of sex hormones. Under normal circumstances, estrogen and androgen receptors exist in breast tissue. The expression of ERs and androgen receptors is reduced or completely disappears when breast epithelial cancer occurs (17,26). In patients with the expression of estrogen and androgen receptors in breast tissue, endocrine therapy is effective, suggesting that these tumors are regulated by the endocrine system (27). In this study, ER-positive and/or PR-positive patients were considered to be HR-positive. The results showed that the risk of BM was relatively higher in HR-positive BC patients. Further analysis showed that the risk of BM was higher in ER-positive BC patients and lower in PR-positive BC patients, which suggests that the PR may be a protective factor against BM in BC patients. The risk of BM may be higher in BC patients who are positive for ER and negative for PR, which is similar to the conclusions drawn by Wei et al. (28).
HER2 expression
HER2 participates in the regulation of cell growth, proliferation, and differentiation. The HER2 gene, also known as the c-erbB-2 gene, is located on chromosome 17q21 and has intracellular tyrosine kinase activity. It is a commonly used gene marker for BC because of its frequent changes after tumor occurrence. Approximately 20–25% of BC cells overexpress HER2 (29) (HER2-positive BC). Compared with other types of BC, HER2-positive BC has distinct clinical characteristics (30), such as a higher risk of recurrence and metastasis and insensitivity to chemotherapeutic drugs (31). Some researchers believe that HER2 overexpression (c-erbB-2 positive) is more useful than HR expression and tumor size for the prognostic evaluation of BC patients, and has a certain correlation with nodal metastasis (32). Ten studies were included in this analysis. The results showed that BC patients with HER2 expression had a relatively higher risk of BM.
Subtype
According to the expression of PR, ER, and HER2, the subtypes of BC can be divided into luminal A, luminal B, HER2, and TBNC. Until now, there have been few studies evaluating the risk of BM in patients with different subtypes of BC, and the results were inconclusive. Yamashiro et al. (24) showed that patients with luminal A BC had the lowest risk of BM, and the risk was significantly lower than that in patients with the other 3 subtypes of BC. In the study by Diessner et al. (12), the highest incidence of BM was in patients with luminal A BC (32.7%). Research data from Xiong et al. (23) showed that the risk of BM in patients with luminal B BC was higher than that in patients with luminal A BC. The risk of BM in patients with HER2 BC and TNBC was lower than that in patients with luminal A. Li et al. showed that there was no difference in the risk of BM among the above 4 subtypes of BC (18). Ossovskaya et al. found that TNBC was characterized by early bone and visceral metastases (33). The results of this study showed that there were no differences among the 4 reports included in this meta-analysis, and it cannot be confirmed whether subtypes have an effect on the BM of BC.
Menopausal status
The menopausal status of women is an important factor affecting sex hormone levels and blood calcium levels. Bisphosphate has a good therapeutic effect on postmenopausal BC patients, suggesting that menopausal status is directly related to disease progression and the therapeutic effect in BC patients (34). The results of this study showed that the relationship between menopausal status and BM of BC was not significant, which may be due to the close relationship between the menopausal status and factors such as age and sex hormone levels, and the combined effect of multiple factors is unclear (35).
Histological grade
The histological grade of BC reflects the invasive ability of the tumor cells. The histological grade of BC is related to recurrence, metastasis, and prognosis (36). In this paper, data on the tumor grade from 8 studies were included in the meta-analysis. The results showed that the incidence of BM in patients with grade 2 and grade 3 cancer were not significantly different from that in patients with grade 1 cancer. Reports from Harries et al. (15), Liede et al. (19), and Xiong et al. (23) found that the incidence rates of BM in patients with grade 2 and grade 3 cancer were significantly higher than in patients with grade 1 cancer, while other studies showed no significant differences. This suggests that histological grade is not suitable for evaluating the risk of BM in BC.
Nodal metastasis
Axillary nodal metastasis is closely related to local recurrence, recurrence time, and distant metastasis in BC patients. The metastatic routes of BC are mainly lymphatic metastasis, blood flow metastasis, and direct invasion. Tumor cells can enter the blood circulation directly or through the lymphatic route to transfer to the lung, bone, and liver. Approximately 50–75% of the lymph is drained through the axillary lymph duct, which is why BC patients are relatively more prone to axillary nodal metastasis. In addition, the inhibition of cancer cells by regional lymph nodes depends on the immune function of the body and the number and invasiveness of cancer cells. Nodal metastasis reflects the immune function of the body to a certain extent, so if the number of axillary nodal metastases increases, the chance of distant metastasis of BC increases. A retrospective analysis showed that the incidence of BM in patients with metastatic lymph nodes was significantly higher than that in patients with no lymph node metastasis within 5 years after surgery (37). Rosa et al. (38) defined the number of lymph node metastases as an indicator, and the number of metastases that was predictive of early bone and visceral metastasis was 1–3. Han et al. (39) found that the number of lymph nodes had good predictive value for postoperative recurrence and metastasis after balancing the tumor size and HR expression. The International BC Study Group (IBCSG) (40) reported that when the number of axillary nodal metastases was ≥4, 12.2% had bone metastases within the first 2 years after the operation, and 26.8% had bone metastases within 10 years after the operation. The results of this study showed that the risk of BM in BC patients with lymph node metastasis was 3.65 times higher than that in patients without lymph node metastasis. There was no significant difference in the incidence of BM between BC patients with ≥3 metastatic lymph nodes and those with 0–2 metastatic lymph nodes. This suggests that the occurrence of nodal metastasis is a risk factor for BM in BC patients and the cutoff value of ≥3 is suitable for evaluating the prognosis of BC patients, but not for evaluating the risk of BM.
Tumor size
Tumor size is an independent risk factor for the prognosis of BC patients (41,42), and patients with larger tumors are more prone to relapse and metastasis. The reason is that tumor growth is accompanied by the formation of new blood vessels, and these new blood vessels often lack complete basement membranes, form arteriovenous anastomosis or blind ends, and are distorted in shape. Their capillary permeability is also higher than that of normal vessels. Brown et al. suggested that this mechanism may be involved in BM (43). Whether the tumor diameter exceeds 2 cm is one of the parameters considered in the diagnosis and treatment of BC (44). In this study, the criteria for stratification and statistical methods in the 3 reports on tumor size evaluation were different. Yamashiro et al. (24) showed that the larger the tumor was, the higher the risk of BM, based on continuous data analysis. Li et al. (18) and Harries et al. (15) divided the tumor size into 3 levels using the cutoffs of 2 and 5 cm. The results showed that the risk of BM in patients with a tumor diameter of 2–5 cm was higher than that in patients with a tumor diameter less than 2 cm, and the risk of BM was higher in patients with a tumor diameter above 5 cm. Liede et al. (19) further stratified the patients by tumor diameter and found that the risk of BM was higher in patients with tumor diameters of 1.1–2 cm than in patients with diameters ≤1 cm, while the risk of BM was higher in patients with diameters of 2.1–5 and ≥5 cm. James et al. (17) showed that in stage IV BC patients, the larger the tumor diameter was, the lower the incidence of BM. The prognosis of BC patients with bone metastases is generally better than that of patients with metastases of other organs. This result suggests that tumor size has good predictive value for the prognosis of BC patients and is suitable for the prediction of BM in patients with stage I to stage III BC. The predictive value may be reduced in patients with stage IV BC.
T stage
In TNM staging of BC, T stage is classified mainly based on tumor size. Among them, T0 is tumor-free, a tumor diameter of ≤2 cm is classified as T1, a tumor diameter of 2–5 cm is classified as T2, a tumor diameter of >5 cm is classified as T3, and the T4 tumor directly affects the chest and/or the skin, regardless of the size of the tumor. Compared with tumor size, T4 increases the ability of T stage to judge the clinical invasion of BC (45). The results of the meta-analysis of 5 studies included in this study showed that the risks of bone metastases in patients with stages T2, T3, and T4 BC were 1.99 times, 4.74 times, and 14.57 times that of stage T1 BC patients, respectively. At the same time, it also suggests that the higher the T stage is, the higher the risk of BM. Compared with tumor size, T stage is more useful for predicting the BM of BC.
Pathological types
The pathological types of BC include invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), mixed carcinoma, mucinous carcinoma, and papillary carcinoma, among which IDC and ILC account for the majority. Some studies have found that the proportion of ILC patients positive for ER and PR was higher than the proportion of IDC patients positive for ER and PR. Fewer patients with ILC than with IDC were positive for HER2, and the tumor volume was larger in ILC patients than in IDC patients (46). Therefore, it is generally believed that BM is relatively more common in ILC patients. In this meta-analysis, 6 studies compared the incidence of BM in patients with ILC and IDC. The results showed that there was no significant difference. However, 4 studies compared the risk of BM in BC patients with pathological types other than ILC and IDC (such as mixed type, mucinous cancer, and papillary cancer) and found that the risk was higher than in patients with IDC.
Research limitations
This study has the following limitations: (I) although 18 articles were included in this study, only HER2 and lymph node metastasis were analyzed in ≥10 studies, thereby increasing the heterogeneity, and Begg’s rank correlation test results indicated the presence of bias. (II) The original data were reported as the number and OR (HR). After the data conversion process, the meta-analysis was performed with OR and 95% CI. The conversion of some data (such as tumor size) could have produced errors. (III) For comparisons among multiple sets of data, considering the small number of included studies, this study adopted the subgroup analysis method, i.e., performed a comparative analysis with the same control group. However, a meta-analysis was not performed, and comprehensive comparisons were not made. (IV) Given the large fluctuations in serological indicators and the small sample size for the genetic factors, serological indicators and genetic factors associated with BM from BC were not included in this study. (V) The inclusion criteria of this study were commonly used to evaluate the risk of distant metastasis in BC patients. At present, there were few studies on specific biomarkers of BM, which is not enough for systematic evaluation. BC is prone to BM, but the prognosis of patients with BM is better than that of patients with lung metastasis and brain metastasis. Therefore, the index screening for the recurrence, metastasis, and prognosis of BC patients is different from the evaluation of BM. The indicators included in this study have mostly been confirmed to have a strong relationship with the prognosis of BC patients. However, the meta-analysis results showed that ER, HR, menopausal status, molecular type, and histological grade were not closely related to the occurrence of BM. PR-positive BC patients have a relatively lower risk of BM. Patients with HER2-positive, nonlobular, and nonductal BC with metastatic lymph nodes have a relatively higher risk of BM. Nodal metastasis can be regarded as a risk factor for BM in BC patients, and ≥3 metastatic lymph nodes was a suitable cutoff value for evaluating the prognosis of BC patients, but not for evaluating the risk of BM. With increasing T stage, the risk of BM in BC patients also increases.
Acknowledgments
Funding: Supported by Capital Characterized Clinical Application Research Fund of Beijing Municipal Science and Technology Commission of China (Z171100001017210); Beijing Hope Run Special Fund of Cancer Foundation of China (LC2016L01); CAMS Innovation Fund for Medical Sciences (CIFMS) (2017-I2M-1-005).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist http://dx.doi.org/10.21037/apm-21-438
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-438). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Body JJ, Quinn G, Talbot S, et al. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit Rev Oncol Hematol 2017;115:67-80. [Crossref] [PubMed]
- Wu Q, Li J, Zhu S, et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget 2017;8:27990-6. [Crossref] [PubMed]
- Leone BA, Vallejo CT, Romero AO, et al. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res Treat 2017;161:537-48. [Crossref] [PubMed]
- Awan AA, Hutton B, Hilton J, et al. De-escalation of bone-modifying agents in patients with bone metastases from breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 2019;176:507-17. [Crossref] [PubMed]
- Yang M, Yu X. Management of bone metastasis with intravenous bisphosphonates in breast cancer: a systematic review and meta-analysis of dosing frequency. Support Care Cancer 2020;28:2533-40. [Crossref] [PubMed]
- Zhang H, Zhu W, Biskup E, et al. Incidence, risk factors and prognostic characteristics of bone metastases and skeletal-related events (SREs) in breast cancer patients: A systematic review of the real world data. J Bone Oncol 2018;11:38-50. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Chen J, Zhu S, Xie XZ, et al. Analysis of clinicopathological factors associated with bone metastasis in breast cancer. J Huazhong Univ Sci Technolog Med Sci 2013;33:122-5. [Crossref] [PubMed]
- Chen X, Sun L, Cong Y, et al. Baseline staging tests based on molecular subtype is necessary for newly diagnosed breast cancer. J Exp Clin Cancer Res 2014;33:28. [Crossref] [PubMed]
- Chen WZ, Shen JF, Zhou Y, et al. Clinical characteristics and risk factors for developing bone metastases in patients with breast cancer. Sci Rep 2017;7:11325. [Crossref] [PubMed]
- Delpech Y, Bashour SI, Lousquy R, et al. Clinical nomogram to predict bone-only metastasis in patients with early breast carcinoma. Br J Cancer 2015;113:1003-9. [Crossref] [PubMed]
- Diessner J, Wischnewsky M, Stuber T, et al. Evaluation of clinical parameters influencing the development of bone metastasis in breast cancer. BMC Cancer 2016;16:307. [Crossref] [PubMed]
- Cronin-Fenton D, Kjaersgaard A, Norgaard M, et al. Breast cancer recurrence, bone metastases, and visceral metastases in women with stage II and III breast cancer in Denmark. Breast Cancer Res Treat 2018;167:517-28. [Crossref] [PubMed]
- Gong Y, Zhang J, Ji P, et al. Incidence proportions and prognosis of breast cancer patients with bone metastases at initial diagnosis. Cancer Med 2018;7:4156-69. [Crossref] [PubMed]
- Harries M, Taylor A, Holmberg L, et al. Incidence of bone metastases and survival after a diagnosis of bone metastases in breast cancer patients. Cancer Epidemiol 2014;38:427-34. [Crossref] [PubMed]
- Irawan C, Hukom R, Prayogo N. Factors associated with bone metastasis in breast cancer: a preliminary study in an Indonesian population. Acta Med Indones 2008;40:178-80. [PubMed]
- James JJ, Evans AJ, Pinder SE, et al. Bone metastases from breast carcinoma: histopathological - radiological correlations and prognostic features. Br J Cancer 2003;89:660-5. [Crossref] [PubMed]
- Li Q, Chen Z, Zhao Y, et al. Risk of metastasis among rib abnormalities on bone scans in breast cancer patients. Sci Rep 2015;5:9587. [Crossref] [PubMed]
- Liede A, Jerzak KJ, Hernandez RK, et al. The incidence of bone metastasis after early-stage breast cancer in Canada. Breast Cancer Res Treat 2016;156:587-95. [Crossref] [PubMed]
- Mihai R, Stevens J, McKinney C, et al. Expression of the calcium receptor in human breast cancer--a potential new marker predicting the risk of bone metastases. Eur J Surg Oncol 2006;32:511-5. [Crossref] [PubMed]
- von Moos R, Body JJ, Rider A, et al. Bone-targeted agent treatment patterns and the impact of bone metastases on patients with advanced breast cancer in real-world practice in six European countries. J Bone Oncol 2017;11:1-9. [Crossref] [PubMed]
- Valsecchi ME, Pomerantz SC, Jaslow R, et al. Reduced risk of bone metastasis for patients with breast cancer who use COX-2 inhibitors. Clin Breast Cancer 2009;9:225-30. [Crossref] [PubMed]
- Xiong Z, Deng G, Huang X, et al. Bone metastasis pattern in initial metastatic breast cancer: a population-based study. Cancer Manag Res 2018;10:287-95. [Crossref] [PubMed]
- Yamashiro H, Takada M, Nakatani E, et al. Prevalence and risk factors of bone metastasis and skeletal related events in patients with primary breast cancer in Japan. Int J Clin Oncol 2014;19:852-62. [Crossref] [PubMed]
- Yazdani A, Dorri S, Atashi A, et al. Bone Metastasis Prognostic Factors in Breast Cancer. Breast Cancer (Auckl) 2019;13:1178223419830978 [Crossref] [PubMed]
- Metzger-Filho O, Sun Z, Viale G, et al. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol 2013;31:3083-90. [Crossref] [PubMed]
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271-7. [Crossref] [PubMed]
- Wei B, Wang J, Bourne P, et al. Bone metastasis is strongly associated with estrogen receptor-positive/progesterone receptor-negative breast carcinomas. Hum Pathol 2008;39:1809-15. [Crossref] [PubMed]
- Howard EM, Lau SK, Lyles RH, et al. Correlation and expression of p53, HER-2, vascular endothelial growth factor (VEGF), and e-cadherin in a high-risk breast-cancer population. Int J Clin Oncol 2004;9:154-60. [Crossref] [PubMed]
- Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 2007;18:1133-44. [Crossref] [PubMed]
- DiGiovanna MP, Stern DF, Edgerton S, et al. Influence of activation state of ErbB-2 (HER-2) on response to adjuvant cyclophosphamide, doxorubicin, and fluorouracil for stage II, node-positive breast cancer: study 8541 from the Cancer and Leukemia Group B. J Clin Oncol 2008;26:2364-72. [Crossref] [PubMed]
- Disis ML, Schiffman K. Cancer vaccines targeting the HER2/neu oncogenic protein. Semin Oncol 2001;28:12-20. [Crossref] [PubMed]
- Ossovskaya V, Wang Y, Budoff A, et al. Exploring molecular pathways of triple-negative breast cancer. Genes Cancer 2011;2:870-9. [Crossref] [PubMed]
- Pulido C, Vendrell I, Ferreira AR, et al. Bone metastasis risk factors in breast cancer. Ecancermedicalscience 2017;11:715. [Crossref] [PubMed]
- Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer 1998;77:336-40. [Crossref] [PubMed]
- Clayforth C, Fritschi L, McEvoy SP, et al. Five-year survival from breast cancer in Western Australia over a decade. Breast 2007;16:375-81. [Crossref] [PubMed]
- Ogura H, Ohya K. Physiology and pharmacology of hard tissues--effect of chemicals on the formation and the resorption mechanism of tooth and bone. Nihon Yakurigaku Zasshi 1995;105:305-18. [Crossref] [PubMed]
- Rosa Mendoza ES, Moreno E, Caguioa PB. Predictors of early distant metastasis in women with breast cancer. J Cancer Res Clin Oncol 2013;139:645-52. [Crossref] [PubMed]
- Han HH, Lee SH, Kim BG, et al. Estrogen Receptor Status Predicts Late-Onset Skeletal Recurrence in Breast Cancer Patients. Medicine (Baltimore) 2016;95:e2909 [Crossref] [PubMed]
- Colleoni M, O'Neill A, Goldhirsch A, et al. Identifying breast cancer patients at high risk for bone metastases. J Clin Oncol 2000;18:3925-35. [Crossref] [PubMed]
- Petracci E, Decarli A, Schairer C, et al. Risk factor modification and projections of absolute breast cancer risk. J Natl Cancer Inst 2011;103:1037-48. [Crossref] [PubMed]
- Hilsenbeck SG, Ravdin PM, de Moor CA, et al. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat 1998;52:227-37. [Crossref] [PubMed]
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res 1998;58:1408-16. [PubMed]
- Cuzick J, DeCensi A, Arun B, et al. Preventive therapy for breast cancer: a consensus statement. Lancet Oncol 2011;12:496-503. [Crossref] [PubMed]
- Fouad TM, Barrera AMG, Reuben JM, et al. Inflammatory breast cancer: a proposed conceptual shift in the UICC-AJCC TNM staging system. Lancet Oncol 2017;18:e228-32. [Crossref] [PubMed]
- Williams LA, Hoadley KA, Nichols HB, et al. Differences in race, molecular and tumor characteristics among women diagnosed with invasive ductal and lobular breast carcinomas. Cancer Causes Control 2019;30:31-9. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)












