
HER2 exon 20 insertion mutations in lung adenocarcinoma with leptomeningeal metastasis: a case report and response to poziotinib
Introduction
Leptomeningeal metastasis (LM) is a specific type of neurological metastasis of lung cancer. Meningeal metastasis occurs in 3% to 5% of patients and are associated with a dismal prognosis (1). Due to the existence of the blood-brain barrier, it is difficult for traditional chemotherapeutic drugs to enter the space between the pia mater and brain parenchyma. Patients with LM usually have a poor prognosis and the 1-year survival rate is less than 10% (2). However, with the advent of the era of precision therapy, the overall survival of non-small cell lung cancer (NSCLC) patients with targeted gene mutations has been improved. Tyrosine kinase inhibitor (TKI) provides significant clinical benefits for most cancer patients with “classic” sensitized mutations (including deletions in exon 19 and the mutation encoding p.L858R). LM is more common in people with EGFR mutations than wild-type epidermal growth factor receptor (EGFR) mutations in lung adenocarcinoma (3) and about 43–70.5% LM-patient are EGFR-sensitive mutations (4-6). EGFR-TKIs is one of the predictors of good prognosis for LM-patients (7) and can prolong the survival time of LM-patients with EGFR positive (5,6,8). Human epidermal growth factor receptor 2 (HER2/ERBB2) as a target remains poorly described, partly because of its lower incidence occurring in 1.7–3.33% without potent targeted therapies among reported lung cancer biomarkers (9-11). In this study, HER2 exon 20 insertion mutations were found in LM-patient of the lung adenocarcinomas and sensitive to poziotinib. We present the following article in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-213/rc).
Case presentation
A 55-year-old male with a history of smoking developed chest tightness in March 2016. Chest computed tomography (CT) scan showed nodules in the left lung’s lower lobe (Figure 1A). Postoperative pathology showed a mass of about 2.1×1.6×1.6 cm3 of invasive adenocarcinoma (Figure 1B) in the lower lobe of the left lung. The immunohistochemical (IHC) analysis revealed positive staining for thyroid transcription factor-1 (TTF-1) and napsin A and negative for p40, CK5/6, VIM, and LCA. Two genes include EGFR and ROS-1, were detected for ARMS method and mutations were not detected. Then he received vinorelbine combined with carboplatin (NC regimen) for four cycles, and the progression-free survival time (PFS) was 10 months. The patient developed disease progression in March 2017. Positron emission computed tomography (PET-CT) showed bilateral pulmonary fibrous hyperplasia, enlarged lymph nodes adjacent to the left pulmonary artery in the mediastinum, and left adrenal metastasis. Pemetrexed plus carboplatin (AC regimen) combined with bevacizumab for four cycles was initiated on March 29, 2017. However, the patient could not tolerate the adverse reactions caused by chemotherapy due to the poor physical condition. Therefore, pemetrexed was discontinued then followed by 12 cycles of bevacizumab alone for continuation maintenance therapy (CMT). The disease progressed again after 1 year. Chest and upper abdominal CT examination showed an enlargement of mediastinal and left supraclavicular lymph nodes, thickening of the right pleura, and metastasis of the liver left adrenal nodules, and the bone (Figure 1C). The patients were treated with anlotinib for 1 month, and then switched to afatinib for 1 month. In August 2018, the patient received immunotherapy with pembrolizumab for one cycle and then changed to anlotinib combined with nivolumab for three cycles. However, the CT of September 27, 2018 showed that although the left pleural multiple plaques with left pleural effusion was not enlarged, the multiple miliary foci of the lung, left hepatic metastatic lesion, and systemic multiple bone metastatic lesions were significantly increased than before medication (Figure 1D). Furthermore, the patient developed mental symptoms of depression and intermittent aphasia with short-term speech loss after 1 month. Adenocarcinoma cells (Figure 1E) were found in the cerebrospinal fluid obtained by lumbar puncture. Magnetic resonance imaging (MRI) on October 29, 2018 indicated abnormal enhancement on the surface of the left frontal-parietal lobe and metastatic tumors (Figure 1F). Targeted next-generation sequencing (NGS) of the primary lung tumor and the cerebrospinal fluid (CSF) are shown in Table 1 and Figure 2. The patient was treated with poziotinib on October 30, 2018. The symptoms improved significantly after 3 days and no serious adverse events were observed during the oral administration of poziotinib. Unfortunately, the disease aggravated again at the end of December 2018. Then, therapy was discontinued and supportive care was given and the patient died on January 17, 2019. This case report was approved by the Medical Ethics Committee of Zhejiang Cancer Hospital (IRB-2019-9).
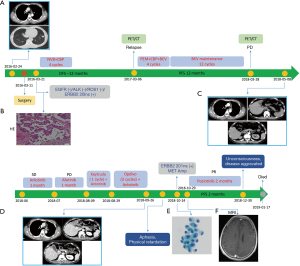
Table 1
| Gene name | Mutation type | Variation | Tissue (2018-04) | CSF (2018-10) | Plasma (2018-10) | ||
|---|---|---|---|---|---|---|---|
| VAF/Change-fold | VAF/Change-fold | VAF/Change-fold | |||||
| ERBB2 | Inframe_insertion | c.2313_2324dupGCATACGTGATG (p.Y772_A775dup) | 16.9% | 44.1% | 22.8% | ||
| TP53 | Frameshift | c.310_311insGAAAACCTACC (p.Q104fs*23) | 16.2% | 97.6% | 46.1% | ||
| TP53 | Missense | c.A299C (p.Q100P) | 19.9% | 97.6% | 46.1% | ||
| TP53 | CNV | One copy loss | WT | 0.6-fold | WT | ||
| MET | CNV | Amplification | WT | 2.3-fold | 1.4-fold | ||
| EGFR | CNV | Amplification | WT | 2.0-fold | 1.6-fold | ||
| HGF | CNV | Amplification | WT | 2.1-fold | WT | ||
| CDK4 | CNV | Amplification | WT | 2.0-fold | WT | ||
| RB1 | CNV | One copy loss | WT | 0.5-fold | WT |
NGS, next-generation sequencing; CNV, copy number variation; WT, wild type.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The question of poor prognosis in LM-patients has become a major issue. Although many techniques have been developed, the next generation sequencing (NGS) of the cerebrospinal fluid (CSF) plays a significant role in the accurate diagnosis, classification, and follow-up treatment of LM-patients (12). The cell-free DNA (cfDNA) of CSF has a unique gene mutation map compared with plasma and primary tissue. In our case, many CNVs were detected in the CSF cfDNA, which were not identified in the primary tissue or plasma, which was consistent with the previous study (13). MET amplification and TP53 deletion was also detected indicating that there may be a more complex mechanism of CSF resistance in LM-patients. The evolution of CNVs may be caused by the genomic instability processes at the chromosomal and mutational levels and even key mutations that predominate in LM-patients (14).
Immunotherapy has been shown to improve survival in patients with NSCLC. Check point inhibitors, nivolumab and pembrolizumab, are recommended as a second line for advanced or metastatic NSCLC. However, there is a great deal of evidence that the patients with EGFR oncogene mutations benefits less from immunotherapy (15-18). In addition to EGFR mutations, ERBB2 activation mutations play a previously unknown role in promoting drug resistance to ICI therapy (19). Patients with EGFR disease and ERBB2 activation mutation who received immunotherapy were negatively correlated with survival time, and their median PFS was lower than that of patients with wild-type gene (log rank P=0.0023) (20). Li et al. (21) reported that none of the seven patients with HER2 mutant NSCLC achieved a response to prior PD-1 inhibitors, which was similar to our patient.
Targeted therapies are highlighted in this small cohort of patients with HER2 exon 20 insertion mutations. The symptoms of LM were relieved rapidly after 3 days of administration of poziotinib and the PFS was nearly 2 months. The patient might derive more clinical benefit if he was treated at an earlier point of the disease course with a better performance status suggesting its specific clinical effect on LM-patients with ERBB2 20 exon insertion. Thus, poziotinib has a unique clinical effect on LM patients with ERBB2 exon 20 insertion. What’s more, poziotinib is a potent tyrosine kinase inhibitor (TKI) of EGFR and HER2 exon 20 insertion mutants (22,23). In a phase II trial of poziotinib, the objective response rate (ORR) of 11 NSCLC patients with EGFR exon 20 mutations was 64% and the median progression-free survival (PFS) duration had not been reached at 6.6 months. These results contrast with ORRs of <10% and PFS durations of <2 months observed when such patients have received approved EGFR TKIs (24). In a phase II study of poziotinib (25), the disease control rate (DCR) was 68.7% and the mPFS was 4.2 months of 115 NSCLC patients with EGFR20 exon insertion mutation. More importantly, the ORR was 8.3% in patients with brain metastasis and 15.5% in patients without brain metastasis.
Nevertheless, there is no report and relevant clinical data analysis about LM-patient with Her2 mutant treated with poziotinib. Previous studies have shown that erlotinib can be more effective for LM than gefitinib due to its higher CSF concentration (26) and the clinical symptoms was obviously improved by using high- dose erlotinib pulse therapy (27,28). Thus, the therapeutic effect can be expected if sufficient TKIs concentration can be achieved in CSF. The CSF concentration and penetration rate of poziotinib may significantly higher than of other TKIs so that it achieves a higher CSF concentration than standard dosing, and successfully controlled LM. However, the CSF concentrations of poziotinib and other TKIs have never been directly compared.
To conclude, this is the first report of a LM-patient who harbored HER2 exon 20 insertion mutations and response to poziotinib. Poziotinib may provide a new therapeutic option for LM-patient and may be especially who are lung adenocarcinoma with HER2 exon 20 insertion. The specific mechanisms keep unclear and large prospective studies should be performed.
Acknowledgments
The authors would like to thank MedSCI editing for editing the language of this paper.
Funding: Funding for this work was provided by the Zhejiang Province Medical Science and Technology Project (No. 2019ZH019) and CSCO-Haosen Cancer Research Foundation (Y-HS2017-044).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-213/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-213/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: https://apm.amegroups.com/article/view/10.21037/apm-21-213/coif). All authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This case report was approved by the Medical Ethics Committee of Zhejiang Cancer Hospital (IRB-2019-9). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Remon J, Le Rhun E, Besse B, et al. Leptomeningeal carcinomatosis in non-small cell lungcancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev 2017;53:128-37. [Crossref] [PubMed]
- Reddy BY, Lim PK, Silverio K, et al. The Microenvironmental Effect in the Progression, Metastasis, and Dormancy of Breast Cancer: A Model System within Bone Marrow. Int J Breast Cancer 2012;2012:721659. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal Metastases in Patients with NSCLC with EGFR Mutations. J Thorac Oncol 2016;11:1962-9. [Crossref] [PubMed]
- Umemura S, Tsubouchi K, Yoshioka H, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer 2012;77:134-9. [Crossref] [PubMed]
- Riess JW, Nagpal S, Iv M, et al. Prolonged survival of patients with nonsmall- cell lung cancer with leptomeningeal carcinomatosis in the modern treatment era. Clin Lung Cancer 2014;15:202-6. [Crossref] [PubMed]
- Lee SJ, Lee JI, Nam DH, et al. Leptomeningeal carcinomatosis in nonsmall- cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol 2013;8:185-91. [Crossref] [PubMed]
- Liao BC, Lee JH, Lin CC, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for Non-Small-Cell Lung Cancer Patients with Leptomeningeal Carcinomatosis. J Thorac Oncol 2015;10:1754-61. [Crossref] [PubMed]
- Xu Q, Chen X, Qian D, et al. Treatment and prognostic analysis of patients with leptomeningeal metastases from non-small cell lung cancer. Thorac Cancer 2015;6:407-12. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012;18:4910-8. [Crossref] [PubMed]
- Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997-2003. [Crossref] [PubMed]
- Jiang BY, Li YS, Guo WB, et al. Detection of Driver and Resistance Mutations in Leptomeningeal Metastases of NSCLC by Next-Generation Sequencing of Cerebrospinal Fluid Circulating Tumor Cells. Clin Cancer Res 2017;23:5480-8. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol 2018;29:945-52. [Crossref] [PubMed]
- Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2109-21. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol 2018;36:633-41. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Fang W, Ma Y, Yin JC, et al. Comprehensive Genomic Profiling Identifies Novel Genetic Predictors of Response to Anti-PD-(L)1 Therapies in Non-Small Cell Lung Cancer. Clin Cancer Res 2019;25:5015-26. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR in hibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Li BT, Shen R, Buonocore D, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol 2018;36:2532-37. [Crossref] [PubMed]
- Sacher A, Le X, Cornelissen R, et al. 36MO Safety, tolerability and preliminary efficacy of poziotinib with twice daily strategy in EGFR/HER2 Exon 20 mutant non-small cell lung cancer. Ann Oncol 2021;32:S15. [Crossref]
- Yang J, Yang J, Ban S, et al. Successful Treatment of a Miliary Metastatic NSCLC Patient With Activating EGFR Exon 20 Insertion Mutation with Response to Poziotinib. J Thorac Oncol 2019;14:e198-e200. [Crossref] [PubMed]
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. [Crossref] [PubMed]
- Socinski MA, Cornelissen R, Garassino MC, et al. LBA60 ZENITH20, a multinational, multi-cohort phase II study of poziotinib in NSCLC patients with EGFR or HER2 exon 20 insertion mutations. Ann Oncol 2020;31:S1188. [Crossref]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Kuiper JL, Smit EF. High-dose, pulsatile erlotinib in two NSCLC patients with leptomeningeal metastases--one with a remarkable thoracic response as well. Lung Cancer 2013;80:102-5. [Crossref] [PubMed]
- Clarke JL, Pao W, Wu N, et al. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Clin Oncol 2011;29:abstr2031.


