
A retrospective analysis of prognostic factors for 160 patients with stage III small cell lung cancer
Introduction
Cases of small cell lung cancer (SCLC) account for 13–17% of all lung cancer cases. SCLC is highly invasive (1), and limited-stage SCLC cases account for 20–30% of the total number of SCLC. According to the American Veterans Association’s two-stage system, SCLC was previously divided into limited and extensive stages. With the development of surgery, precision radiotherapy, and clinical research, SCLC is also staged according to the American Joint Committee on Cancer (AJCC) TNM staging system. Previous studies showed age, smoking history, physical status, size of the tumor, local lymph node metastasis, and different treatment methods might affect SCLC prognosis (2,3). At present, stage III SCLC follows the treatment mode of early chest radiotherapy, four-course chemotherapy, and preventative brain radiation, just like stages I and II SCLC (4). However, some local advanced SCLC recurs and metastasizes early after radiotherapy, which causes the patients economic and psychological pressure. In addition, adverse reactions after radiotherapy often make subsequent chemotherapy difficult. Clinicians are unsure whether the treatment mode of systemic chemotherapy followed by radiotherapy benefits patients, and there is no study on this at present (4). This study retrospectively evaluated prognostic factors for stage III SCLC patients to explore the best treatment mode of locally advanced SCLC. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-50).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Jilin Provincial Cancer Hospital ethics committee (No. 202012-50-01) and informed consent was taken from all the patients.
Subjects
A total of 160 patients with stage III SCLC diagnosed by histology and cytology were included in the analysis. Before treatment, all patients underwent strict staging examinations, including lung and abdomen computed tomography (CT), head magnetic resonance imaging (MRI), and whole-body bone scans. The patients with pleural effusion were examined by exfoliative cytology who did not cancer met the criteria for chemotherapy and radiotherapy, and tumor evaluation was conducted every week.
The inclusion and exclusion criteria: the diagnosis was SCLC histologically or cytologically, according to the American Veterans Association classification of limited-stage SCLC; ECOG PS 0–1; according to RECIST standard, there were measurable lesions; there were no contraindications to chemotherapy and radiotherapy. Patients with a history of heart disease and other chronic diseases were excluded. Finally, 103 patients at stage IIIA and 57 patients at stage IIIB were enrolled. Clinical characteristics of these patients are presented in Table 1.
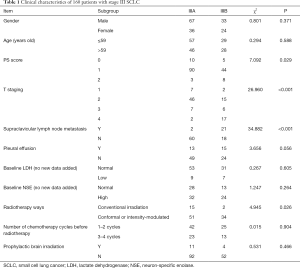
Full table
Therapeutic methods
A total of 160 patients with stage III SCLC were enrolled. According to the AJCC 2007 stage, these patients were classified as stage IIIA or IIIB, including 103 patients at stage IIIA and 57 patients at stage IIIB. The treatment methods included chemotherapy combined with radiotherapy in 107 patients and chemotherapy alone in 53 patients. The first-line application regimens included EP and EC regimens. The patients were divided into two groups according to the start time of chest radiotherapy. In the early radiotherapy group (n=63), radiotherapy was carried out one to two weeks after chemotherapy. In the late radiotherapy group (n=36), the patients were treated with chest radiotherapy after three to four chemotherapy cycles. Methods of chest radiotherapy included 6MV-X-ray intensity-modulated field radiotherapy and chest involved field intensity-modulated radiotherapy. Only 15 patients were treated with prophylactic cranial irradiation (PCI).
Outcomes, clinical variables, and follow-up procedures
The short-term efficacy was evaluated according to RECIST 1.1 solid tumor efficacy evaluation criteria: complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). For assessment of long-term outcomes, the patients who experienced progression of the cancer 60 days after the last treatment were followed every three months until there was a case censoring or they died. The patients who experienced no progression of the cancer and did not get any other anti-tumor treatment were followed every four weeks until they experienced progression of the cancer, died, or there was case censoring. The response rate (RR) = CR + PR. Progression-free survival (PFS) was the time from the beginning of treatment to the disease’s progression, and the overall survival (OS) time was from the beginning of treatment to the last follow-up or death. The clinical variables assessed included PS score, stage, pleural effusion, first-line treatment cycle, therapy effect, radiotherapy methods, baseline lactate dehydrogenase (LDH), baseline neuron-specific enolase (NSE), and the number of chemotherapy cycles before radiotherapy.
Statistic analysis
Statistical analysis was conducted using statistical software SPSS 13.0. Comparison between patients in stages IIIA and IIIB were conducted using chi-square tests. The survival analysis was conducted using the Kaplan-Meier method. Univariate analysis was conducted for all factors in Tables 2 and 3. Significant variables in univariate analysis were introduced into the Cox regression model. P<0.05 (two-sided) was considered statistically significant.
Full table
Full table
Results
Comparison of the short-term and long-term efficacy of different stages of SCLC
All 160 patients were treated with chemotherapy or chemotherapy combined with radiotherapy. The short-term and long-term effects of stages IIIA and IIIB are shown in Table 4. Stage IIIA presented a higher CR rate than stage IIIB. The short-term remission rate was 80.58% in stage IIIA and 85.96% in stage IIIB, and the difference between the two was not statistically significant. The median PFS and OS of stages IIIA and IIIB were 8.5 vs. 9 and 12.5 vs. 14 months, respectively. There was no significant difference in the median PFS and OS between the two groups.

Full table
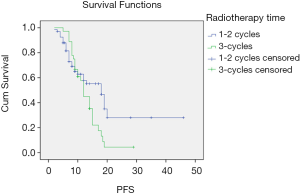
Survival comparison of patients with stage III SCLC with different treatment modes
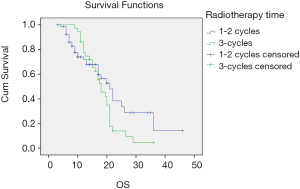
There was no significant difference in PFS between the early radiotherapy and late radiotherapy in stage IIIA SCLC (P=0.065) or OS (P=0.231). PFS was longer in the late radiotherapy group than in the early radiotherapy stage IIIB patients (P=0.041). The difference in OS was not statistically significant between the two groups (P=0.110). The survival curves of different treatment modes are shown in Figures 1 and 2.
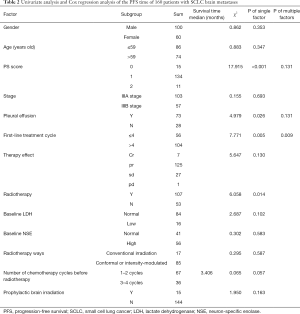
Univariate analysis and Cox regression analysis of PFS in patients with stage III SCLC
The factors related to the survival time of 160 patients with stage III SCLC were compared. Univariate analysis demonstrated that the ECOG PS score, the presence of pleural effusion, first-line chemotherapy cycles, and chest radiotherapy were the main factors affecting PFS. Cox analysis showed that only the number of first-line chemotherapy cycles affects PFS (P=0.009). There was no significant correlation between PFS and gender, age, stage, baseline LDH, and NSE (Table 3).
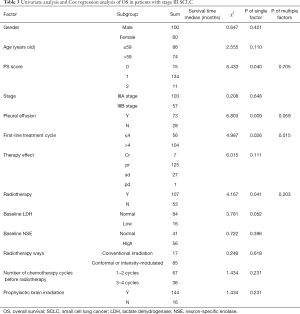
Univariate analysis and Cox regression analysis of OS in patients with stage III SCLC
The factors affecting OS in patients with stage III SCLC included ECOG PS scores, pleural effusion, the number of first-line chemotherapy cycles, and radiotherapy. Cox analysis revealed that ECOG PS scores, pleural effusion, the number of first-line chemotherapy cycles, and chest radiotherapy were independent prognostic factors affecting patients’ OS time with stage III SCLC (Table 4).
Discussion
The cases of SCLC account for 12–15% of the total number of lung cancer patients. The incidence of SCLC in developed countries, such as the USA, has decreased yearly, but China’s incidences are still rising. Compared with non-SCLC, its biological behavior is worse, the doubling time is short, the disease develops rapidly, and it is easy to metastasize early. Although it has a high sensitivity to radiotherapy and chemotherapy, it is easy to relapse and metastasize and has a poor prognosis.
The staging of SCLC adheres to the American Veterans Association’s two-stage system. The limited stage is defined as tumor location on one side of the chest, including ipsilateral mediastinal lymph nodes and bilateral supraclavicular lymph nodes. SCLC, like other malignant tumors, adheres to the principle of staging treatment. With the development of surgery and precision radiotherapy, the American Veterans Association’s two-stage method can no longer meet stratified treatment needs. Therefore, SCLC is currently diagnosed according to the eighth edition TNM staging of lung cancer of the International Association for the Study of Lung Cancer (IASLC). All cases were collected before 2016, according to IASLC’s seventh edition of TNM staging of lung cancer (5). There is no detailed treatment plan for limited-stage SCLC in the two editions. According to the current National Comprehensive Cancer Network (NCCN) guidelines of the United States and the Department of Health’s lung cancer diagnosis and treatment standards, patients with limited-stage SCLC should be treated with concurrent or sequential chemoradiotherapy, and chest radiotherapy should be given after one to two cycles of chemotherapy. For patients in remission, prophylactic brain irradiation (PCI) is the primary treatment strategy (6). However, it is found that patients with limited-stage SCLC, especially patients with stage III, develop recurrence and metastasis quickly after early radiotherapy and are under financial and psychological pressure. In addition, the adverse reactions after radiotherapy often make subsequent chemotherapy difficult. It makes clinicians doubt whether the treatment mode of systemic chemotherapy followed by radiotherapy benefits the patients. Different clinical factors and treatment methods that affect the prognosis of stage III SCLC deserve further discussion.
Among the clinical factors that may affect limited-stage SCLC, the size of the tumor and local lymph node metastasis may be the most important prognostic factors. In 2007, the IASLC used the seventh edition of Lung Cancer TNM staging to evaluate SCLC’s prognosis (7,8) retrospectively. A total of 12,620 cases were included, of which 8,088 were suitable for TNM staging. The results revealed that survival was significantly correlated with T and N, especially for patients without mediastinal and supraclavicular lymph nodes, and the difference in survival was more significant. Therefore, the IASLC suggests that SCLC patients should be staged using the seventh edition of the TNM staging system.
Although clinical evidence suggests that the survival of patients with limited-stage SCLC has improved due to radiotherapy's involvement, the timing of radiotherapy has always been questioned. The meta-analysis revealed that platinum-based chemotherapy was the most important predictor (2,9). A phase III clinical trial involving 231 patients with limited-stage SCLC showed a similar trend, supporting short SER but no difference in OS (10). According to the current guidelines and the Ministry of Health guidelines for diagnosing and treating lung cancer, radiotherapy is recommended after one to two chemotherapy cycles. However, a phase III trial conducted in South Korea in which the first or third cycles of EP chemotherapy combined with TRT to determine the optimal timing of radiotherapy for limited phase SCLC was published. The results revealed that in the delayed group (n=108), median OS was 26.8 months, in the initial group (n=111), median OS was 24.1 months, and in the delayed group (n=108), median PFS was 11.2 months. In the initial group, the median PFS was 12.4 months, so it is suggested that the delayed group was similar to the initial group in OS and CR. The incidence of febrile neutropenia in the delayed group was lower (11). Our study yielded similar results: there were no significant differences in short-term and long-term therapeutic effects between stages IIIA and IIIB. There were no significant differences in PFS and OS between the early and late radiotherapy groups for stage III patients. Patients with stage IIIB receiving late radiotherapy seemed to have a survival advantage, but the difference was not statistically significant (P=0.549). This suggests that after three to four cycles of chemotherapy, radiotherapy may further reduce the distant metastasis and improve patients’ survival in late-stage or limited-stage, which is worthy of further discussion.
Many factors affect the prognosis of SCLC, including age, smoking history, physical status, and stage (3). In addition, tumor markers are also considered to be related to the prognosis of patients. In patients with high tumor markers, the disease may have metastasized to other organs (12). Univariate analysis in our study demonstrated that for patients with stage III, ECOG PS scores, pleural effusion, first-line chemotherapy cycles, and chest radiotherapy were the main factors affecting PFS. Cox analysis revealed that ECOG PS scores, pleural effusion, number of first-line chemotherapy cycles, and chest radiotherapy were independent prognostic factors affecting the OS time of patients with stage III SCLC, while there was no significant correlation between NSE and prognosis.
Conclusions
In this study, after systemic treatment, the PFS and survival time of patients with locally advanced SCLC at stages IIIA and IIIB were similar. For patients at stage IIIA, PFS and survival time in the late radiotherapy group were similar to that in early radiotherapy. The treatment timing has little correlation with prognosis, but patients at stage IIIB seemed to get more benefit from late radiotherapy. Compared with the patients with few first-line chemotherapy cycles and no chest radiotherapy, multi-course chemotherapy and chest radiotherapy could significantly improve the prognosis. In comparison, patients with lower PS scores and limited-stage SCLC with pleural effusion had a relatively poor prognosis. In this retrospective study, the sample size is small, and the follow-up time is relatively short. Further prospective studies should be carried out to improve the survival and prognosis of locally advanced SCLC.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding: This study was supported by Study on the efficacy and mechanism of antinib combined with chemotherapy or immunotargeted drugs in SCLC (2019J077) and Construction of new drug evaluation technology platform for malignant tumors (2020ZX09201-024).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-50
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-50
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at http://dx.doi.org/10.21037/apm-21-50). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Jilin Provincial Cancer Hospital ethics committee (No. 202012-50-01) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDeri
References
- Oronsky B, Reid TR, Oronsky A, et al. What's New in SCLC? A Review. Neoplasia 2017;19:842-7. [Crossref] [PubMed]
- De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol 2006;24:1057-63. [Crossref] [PubMed]
- Nakazawa K, Kurishima K, Tamura T, et al. Specific organ metastases and survival in small cell lung cancer. Oncol Lett 2012;4:617-20. [Crossref] [PubMed]
- Schmittel A. Controversies in the treatment of advanced stages of small cell lung cancer. Front Radiat Ther Oncol 2010;42:193-7. [Crossref] [PubMed]
- Jett JR, Schild SE, Kesler KA, et al. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e400s-19s.
- Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol 2004;22:4837-45. [Crossref] [PubMed]
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor,node,metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [Crossref] [PubMed]
- Komatsu T, Oizumi Y, Kunieda E, et al. Definitive chemoradiotherapy of limited-disease small cell lung cancer: Retrospective analysis of new predictive factors affecting treatment results. Oncol Lett 2011;2:855-60. [Crossref] [PubMed]
- Pijls-Johannesma M, de Ruysscher D, Vansteenkiste J, et al. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Cancer Treat Rev 2007;33:461-73. [Crossref] [PubMed]
- Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent vs. sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054-60. [Crossref] [PubMed]
- Sun JM, Ahn YC, Choi EK, et al. Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann Oncol 2013;24:2088-92. [Crossref] [PubMed]
- Shibayama T, Ueoka H, Nishii K, et al. Complementary roles of pro-gastin-releasing peptide (ProGRP) and neuron specific enolase (NSE) in diagnosis and prognosis of small-cell lung cancer (SCLC). Lung Cancer 2001;32:61-9. [Crossref] [PubMed]


