
Shenfu injection for heart failure based on the AMSTAR-2, PRISMA, and GRADE tools
Introduction
Heart failure (HF) is the final stage of cardiovascular disease, which leads to myocardial damage, along with structural and functional problems. The global prevalence of HF is estimated to exceed 37.79 million (1). Although the incidence of HF has remained at a relatively stable level, the rates of rehospitalization and mortality are still high. Globally, 17–45% of hospitalized HF patients die within 1 year after admission, while the majority die within 5 years after admission. The 5-year death rate is similar to that of many cancers (2,3). According to the American Heart Association, the prevalence rate in developed countries is 1–2% in the adult population. In the United States, 5.8 million patients suffered from HF in 2012, which is expected to rise to 8.5 million by 2030, and the medical costs will rise to $70 billion (4-6). According to the report outline of cardiovascular Disease in China [2018], it is estimated that the number of patients with cardiovascular disease is 290 million, including 4.5 million cases of HF (7), and the in-hospital mortality is 5.3% (451/8,516) (8). It has become a major public health problem worldwide.
Currently, the conventional medical treatment for HF includes angiotensin-converting enzyme inhibitors (ACEIs), beta-blockers, mineralocorticoid/aldosterone receptor antagonists (MRAs), diuretics, angiotensin receptor blockers (ARBs), angiotensin receptor neprilysin inhibitor (ARNI), aldosterone antagonists, digitalis and vasodilators and so on, but the shortcomings of western medicine (WM) are prominent, such as persistent high cost, short-term efficacy, and adverse reactions or side effects (6). Traditional Chinese medicine (TCM) has its own specific characteristics and advantages. Among the treatment methods of TCM, TCM injection is used in the treatment of HF and has shown good efficacy (9). Shenfu injection (SFI) is a TCM injection that is widely used in clinical treatment, and is composed of red ginseng and aconite. It can restore the yang and prevent the adversity, replenish and solidify qi, enhance myocardial contractibility, protect myocardium, improve hemodynamics, and regulate heart rate (10); the safety of SFI is very high, and the incidence of adverse drug reactions (ADRs) is 0.076%, 95% confidence interval (CI): (0.045 to 0.108) (11).
At present, from clinical trials to systematic reviews (SRs), many studies have reported the efficacy of treating HF with either a single or combined conventional drug, but the findings have been inconsistent (12,13). As SRs provide the highest quality of evidence that guides clinical decision-making, it is particularly important to evaluate the quality of SRs. The purpose of this study was to evaluate the methodological quality, quality of the literature report, and to grade the main results, so as to understand the current situation and challenges of SR in SFI treatment of HF, and provide ideas for clinical treatment of HF.
Methods
Search strategy
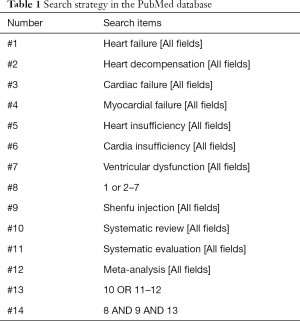
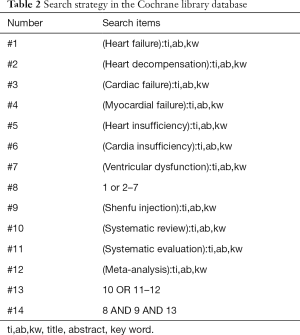
The deadline for literature publication is up to December 2020, relevant literatures were searched for in the following seven databases: Cochrane library, Embase, PubMed, SinoMed, China National Knowledge Infrastructure (CNKI), Wanfang Database, and VIP Database. The key search words included: “Shenfu injection”, “heart failure”, “heart decompensation”, “cardiac failure”, “myocardial failure”, “heart insufficiency”, “cardiac insufficiency”, “ventricular dysfunction”, “systematic review”, “systematic evaluation”, “meta-analysis”. The search strategy was adjusted according to the characteristics of each database. In addition, we manually searched relevant grey literature, conference articles, and other published literature. The literature retrieval was conducted independently by two reviewers (JTJ and ZML). When differences occurred, they were resolved by discussion between the two reviewers, and any unresolved problems were resolved by a third reviewer (FYZ). The search strategies are shown in Tables 1-5.
Full table
Full table

Full table

Full table

Full table
Eligibility criteria
Inclusion criteria
All articles met the following inclusion criteria: (I) must be a SR/meta-analysis (MA) of HF treated by SFI, and each SR must contain at least two randomized controlled trials (RCTs); (II) all participants were diagnosed with HF, and were included regardless of gender, age, region, race, etiology, disease course, degree, and other factors; (III) the treatment group was treated with SFI or combined with other methods, while the control group could be any other treatment method except SFI; (IV) the efficacy indexes included 1 or more of the following: effect rates, left ventricular ejection fraction (LVEF), left ventricular end-diastolic dimension (LVEDd), brain natriuretic peptide (BNP), N-terminal pro-B type natriuretic peptide (NT-proBNP), 6-min walk test (6-MWT), the Minnesota Living With Heart Failure Questionnaire (MLHFQ), mortality, and rehospitalization rate.
Exclusion criteria
All the following literatures were excluded: (I) without SFI treatment; (II) not SR literatures, including animal experiments, RCTs, general reviews, conference articles, case reports, expert consensus, and so on; (III) incomplete data and duplicate papers.
Study selection
The two reviewers (SMC and GZ) strictly followed the eligibility criteria and search strategies to conduct literature searches in the above seven databases without language restrictions. Preliminary screening was carried out through reading titles and abstracts, and the literatures were poured into EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA) to eliminate duplicate documents. Then, all the initial qualified literature titles and were listed and the full text was further read to exclude all non-conforming literature. If there were date defects in the original literature, the original author was contacted. Any dispute was settled between the two reviewers, and any unresolvable differences were settled by a third reviewer (FYZ).
Data extraction
Another two reviewers (JTJ and ZML) extracted data through a unified extraction table, including: general information (title, first author, year of publication, country, language, contact information, funding, conflict of interest, ethical perceptions), research contents (participants, sample size, randomization, allocation concealment, blind, intervention, comparison), research outcomes (risk assessment tools, main outcomes, adverse reactions/events and main conclusions). Any dispute was settled between the two reviewers, and any unresolvable differences were resolved by a third reviewer (FYZ).
Quality assessment
The two reviewers (LYL and SY) independently evaluated all the included SRs according to the requirements of the three evaluation tools [Appraisal Tool for Systematic Reviews of Randomized and Observational Studies 2 (AMSTAR-2) (14-16), Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) (17), and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (18)], and then cross-checked. In the case of inconsistent evaluation results, the two reviewers attempted resolution through discussion, and any unresolved disagreements were settled by a third reviewer (FYZ).
AMSTAR-2
The popular AMSTAR tool was developed in 2007 to rigorously evaluate the methodological quality of SRs. In 2017, AMSTAR-2 was officially published, which can be used to evaluate SRs in randomized or non-randomized trials. AMSTAR-2 retains 10 original items and expands six new items for a total of 16 items. The content of each item is simpler and clearer, and the overall score is based on weaknesses in key areas. Each item requires the reviewers to answer “yes”, “no” and “partial yes”, and items 2, 4, 7, 9, 11, 13, and 15 are key items. It can seriously affect whether the evaluation result is downgraded or not. The quality of the SR is divided into four levels: (I) high: none or only one non-key item does not meet the requirements; (II) moderate: more than one non-key item does not meet the requirements; (III) low: only 1 key item does not meet and it does not meet with or without non-key items; (IV) very low: more than one key item does not meet the requirements, with or without non-key items does not meet the requirements. The AMSTAR-2 tool is consistent with SRs, which indicates that it is a practical method.
PRISMA
The PRISMA tool is used to evaluate reporting quality. The PRISMA statement covers 27 lists. Each item requires the reviewer to answer “yes”, “no”, and “partial yes”. Both AMSTAR-2 and PRISMA can be expressed as a percentage of items that meet “yes”.
GRADE
The GRADE tool is used to grade the quality of evidence of the main outcomes. There may be several reasons for the decrease of evidence, including the study limitations, inconsistency of results, indirectness of evidence, imprecision, or reporting bias. The quality of evidence can be classified into four levels: high, moderate, low, and very low. No degradation equates to high quality, one degradation to moderate quality, two degradation to low quality, and three or more degradation to very low quality.
Statistical analysis
Due to the lack of access to the original data, we could only carry out quantitative synthesis or descriptive analysis of existing data. The kappa index was used to measure reliability between the two reviewers: a kappa index over 0.75 indicated excellent consistency; 0.4–0.75 indicated fair consistency; and less than 0.4 indicated poor consistency.
Results
Search results
According to the search strategy, 103 articles were initially screened from 7 databases, of which 22 were from CNKI, 24 from SinoMed, 25 from Wanfang, 20 from VIP, 8 from Embase, 4 from PubMed, and 0 from Cochrane library. A total of 70 duplicate references were excluded in Endnote X9; further, 8 articles were excluded after reading the titles and abstracts, and then 13 articles were excluded by reading the full text. Finally, a total of 12 articles were included for data analysis (19-30). The process of the literature search and screening is shown in Figure 1. The list of excluded articles is shown in Table 6.

Full table
Characteristics of included reviews
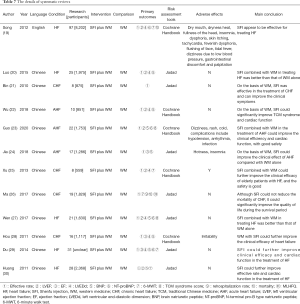
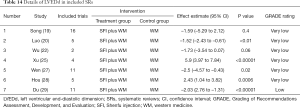
A total of 12 literatures were peer-reviewed articles from 2011 to 2020, 11 of which were published in Chinese journals and 1 in British journals (19). This study involved 302 original RCTs and more than 22,445 participants [1 of which did not mention the total number of participants (29)]. The minimum and maximum sample sizes were 8 RCTs (559 participants) (25) and 97 RCTs (8,202 participants) (19), respectively. The treatment groups were treated with SFI plus WM, and the control groups were treated with WM. The main result was clinical efficacy rate, and the secondary results included LVEF, LVEDd, BNP, NT-proBNP, 6-MWT, MLHFQ, mortality, and rehospitalization rate. All literatures were evaluated by methodology, including 7 by the Jadad scale, and 5 by the Cochrane Handbook. A total of 5 studies reported adverse events (AEs), 1 study reported no adverse reactions (25), and 4 studies delineated specific adverse reactions (19,23,24,28) (see Table 7 for detailed literature features).
Full table
Methodological assessment
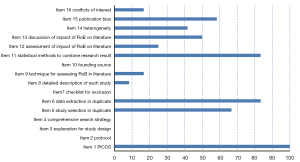
The methodological quality of the literature was evaluated by AMSTAR-2, among which items 2, 4, 7, 9, 11, 13, and 15 were key items. The literature failed to meet any of the requirements and was rated as very low level. The evaluation results showed that the methodological evaluation level of all literatures was very low. First of all, (I) all literature highlighted research problems and inclusion criteria in accordance with the PICO principle. (II) Most literature listed the screening process [except (21,26,30)]. (III) At least two evaluators extracted data independently [except (21,28)]. (IV) Evaluation tools were used to assess the risk of bias in the included studies and the research data were analyzed comprehensively [except (20,30)]. (V) A total of six studies explained the possible causes of the risk of bias (19,22,23,25-27). (VI) A total of seven studies discussed the impact of risk of bias on the results (19,21,23,26-28,30). However, with regard to its key items, (I) there was no preliminary design plan or registration protocol before the SR in all articles. (II) No reproducible and comprehensive search strategy was provided. (III) No detailed exclusion list and exclusion reasons were listed. (IV) The assessment of the risk of bias was not comprehensive [except (19,23)]. The detailed results are shown in Figure 2 and Table 8. The reliability between the two reviewers was excellent (kappa =0.941).
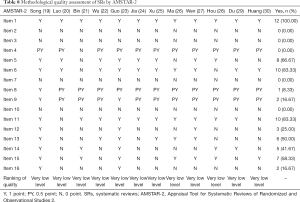
Full table
Reporting quality
Some of the papers were of good quality, with an average score of 18.25 and a completion degree of 13.5–23 points. Among them, the main measures of project title, data synthesis, risk assessment methods, and results of bias were comprehensively reported (100%). Most literatures reported basic principles, qualification criteria, study selection, study characteristics, individual study results, results synthesis, and conclusions (over 75%). There was no pre-registration of literatures, and some of the articles were incomplete or unreported for the remaining entries. Report quality of the studies is shown in Figure 3 and Table 9. The reliability between the two reviewers was excellent (kappa =0.886).
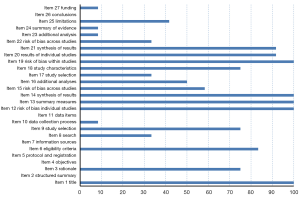
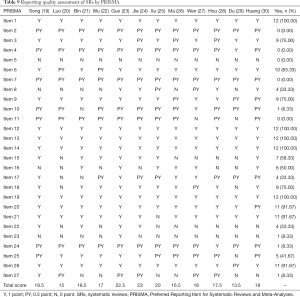
Full table
Quality of evidence
The 12 studies contained a total of 52 outcome indicators, of which 3 were moderate quality, 13 were low quality, and the rest were very low quality. The limitations of all results were reduced, followed by publication bias (48 results), imprecision (29 results), inconsistency (24 results), and indirectness (0 results). The relevant results are shown in Table 10.
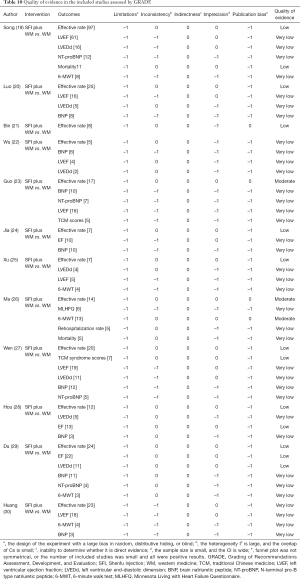
Full table
Outcomes
Effective rate
All literatures analyzed the effective rate, including comprehensive curative effect (20,24,27-29), clinical effective rate (19,21,23,25,26,30), and TCM syndrome curative effect (22). The results suggested that SFI combined with WM could significantly improve the clinical efficacy of HF treatment. The results are shown in Figure 4 and Table 11.
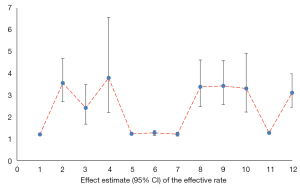
Full table
Cardiac parameters
We counted the related indicators of cardiac function, including LVEF, EF, LVEDd, BNP, NT-proBNP, and 6-MWT. Xu et al. (25) pointed out that LVEF was not statistically significantly different compared with the control group (P=0.05, 5 trials). It was highlighted in two SRs (19,22) that LVEDd was not statistically significant compared with the control group (respectively, P=0.06, 2 trials and P=0.4, 16 trials). Other results suggested that SFI could improve the cardiac function of patients with HF. A total of 7 SRs analyzed LVEF, 3 analyzed EF, 7 analyzed LVEDd, 4 analyzed NT-probNP, and 5 analyzed 6-MWT. The results are shown in Figures 5-10 and Tables 12-17.
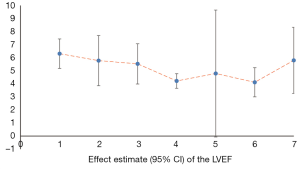
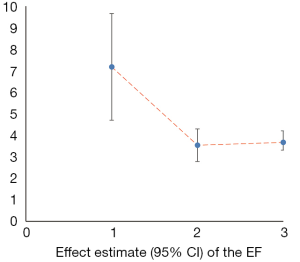

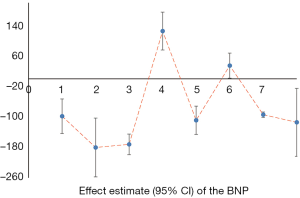
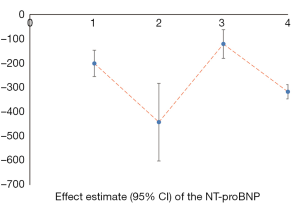
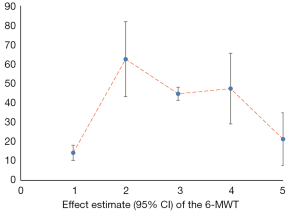

Full table

Full table
Full table
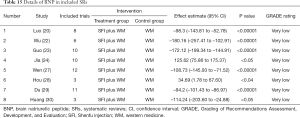
Full table
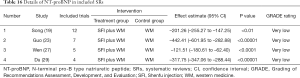
Full table

Full table
Mortality and rehospitalization rate
A total of two SRs analyzed death and conducted a subgroup analyses. It was shown that SFI can significantly reduce the mortality of HF patients induced by myocardial infarction [relative risk (RR) =0.52, 95% CI: 0.37 to 0.74; P<0.01]. In other subgroups, there was no significant difference between the two groups (RR =0.68, 95% CI: 0.36 to 1.26; P=0.22). However, the overall results of the two subgroups were significantly different (RR =0.56, 95% CI: 0.41 to 0.75; P<0.01, 11 trials) (19). Another study found no significant difference in mortality compared with the control group (OR =0.59, 95% CI: 0.31 to 1.13; P>0.05). At the same time, the researchers analyzed the rehospitalization rate, and the difference was statistically significant (OR =0.42, 95% CI: 0.29 to 0.59; P<0.05, 5 trials) (26).
TCM syndrome score and MLHFQ score
A total of two studies (23,27) showed that SFI combined with WM can significantly improve TCM syndrome scores in patients with HF, but the quality of evidence was poor [OR =2.94, 95% CI: 1.71 to 5.04; P<0.0001, 7 trials and mean difference (MD) =–2.12, 95% CI: –2.93 to 1.31; P<0.00001, 5 trials]. Only one study (26) results showed that MLHFQ score was significantly better in the SFI + WM group than in the control group (MD =–5.57, 95% CI: –8.26 to –2.87); P<0.01, 6 trials).
AEs
A total of five studies reported AEs; one study reported no adverse reactions, and four studies described specific adverse reactions. The AEs specifically included: hotness, insomnia (24); dry mouth, dryness heat, fullness of the head, insomnia, dysphoria, skin itching, tachycardia, feverish dysphoria, flushing of face, tidal fever, dizziness due to low blood pressure, gastrointestinal discomfort, and palpitation (19); irritability (28); dizziness, rash, cold, and complications included hypotension, arrhythmia, and infection (23).
Discussion
Summary of main findings
A total of 12 SRs were reported on in this study, and the results showed that SFI combined with WM was more effective than using WM alone for HF. However, most conclusions emphasized that it was necessary to carry out more high-quality RCTs for verification of findings. The results of SR are considered the highest quality of evidence to guide clinical decision-making. We searched SRs related to the treatment of HF with SFI, and comprehensively evaluated the methodological quality, quality of reports in literature, and quality of evidence of main results by using AMSTAR-2, PRISMA, and GRADE evaluation tools. Unfortunately, the methodological quality and the reporting quality were not high in most SRs involved in this study.
The treatment of HF, especially chronic HF (CHF), is a long process. In the future, when conducting studies on SFI in the treatment of HF, researchers should appropriately increase the follow-up time, strengthen the observation of cardiovascular end points, such as rehospitalization rate and mortality (within 1 or 5 years), and pay more attention to improving the quality of the methodology.
Efficacy and safety of SFI in acute phase of CHF
Evidence has shown that SFI was also used for treatment of CHF during the acute phase, but few standard clinical trials have evaluated its efficacy and safety. In 2009, a randomized, double-blind, multicenter, placebo-controlled trial was the first to evaluate the safety and efficacy of SFI in treating patients with CHF in the acute phase (31). Main outcomes included New York Heart Association (NYHA) classification and TCM syndrome scores. NYHA has been shown to be closely associated with survival and is used to reflect the severity of acute HF (AHF) (32,33). TCM syndrome score is based on TCM symptoms and signs. It is one of the most important and commonly used indexes in TCM efficacy evaluation (34). The major results included the following: (I) The clinical symptoms and cardiac tolerance of the patients were improved; (II) SFI did not induce AEs or ADRs. In fact, it is necessary to further explore the efficacy and safety of SFI for CHF patients with acute phase.
Strengths and limitations
Strengths: (I) as far as we know, this study was the first to evaluate the latest overview of HF-related SR treated with SFI in strict accordance with the assessment requirements of AMSTAR-2, PRISMA, and GRADE. (II) We comprehensively searched seven databases and enumerated clear retrieval strategies one by one, which was reproducible. (III) The data synthesis and composition of the main outcome indicators are helpful to more intuitive analysis of the advantages and disadvantages of the research results.
Limitations: (I) the quality of the RCT-based SR methodology and the quality of the literature report was not shown to be high, which limited our judgment of the results to some extent. (II) A total of seven literatures were published earlier than the launch of AMSTAR-2, and the original authors did not follow the existing rules, which may be one of the reasons for the low quality of methodology. (III) Admittedly, this was the first time we had used the AMSTAR-2, PRISMA, and GRADE tools, and our understanding of some items may have been somewhat skewed, but consistency between the two reviewers was ensured as much as possible.
Factors influencing methodological quality and literature reporting quality
Firstly, AMSTAR-2 (16 items) is used to evaluate the methodology quality of the system evaluation, five of which are critical, and failure to perform any of them will result in a direct downgrade, including (14-16):
- Protocol registered before commencement of the review (item 2);
- Adequacy of the literature search (item 4);
- Justification for excluding individual studies (item 7);
- Risk of bias from individual studies being included in the review (item 9);
- Appropriateness of meta-analytical methods (item 11);
- Consideration of risk of bias when interpreting the results of the review (item 13);
- Assessment of presence and likely impact of publication bias (item 15).
In addition, the PRISMA statement consists of a 27-item checklist and a four-phase flow diagram. The checklist includes items deemed essential for transparent reporting of a SR (17). Therefore, whether a reviewer follows the AMSTAR-2 (16 items) and PRISMA (27 items) tools in developing a SR will have a significant impact on methodological quality and literature reporting quality.
Secondly, the quality of the original literature included in the SR/MA, such as RCTs, did not strictly follow the Consolidates Standards of Reporting Trials (CONSORT), which was affect the reporting bias of the SR. Therefore, clinical researchers should conduct high-quality studies in strict accordance with the CONSORT standard in order to obtain more scientific, accurate and high-quality clinical evidence. Finally, we must acknowledge that although the AMSTAR-2 and PRISMA tools have outlined specific evaluation criteria, the tools are interpreted differently by different researchers, subjectively affecting the quality of the methodology and the quality of the reporting results.
AMSTAR-2: an update based on AMSTAR
AMSTAR-2 changed the four items in AMSTAR to five items. Is research selection and data extraction conducted independently by two reviewers? (Item 2 in AMSTAR was split into items 5 and 6). Did the author report the source of funding and any potential conflicts of interest? (Item 11 in AMSTAR was split into items 10 and 16). Whether publication status is adequately considered in inclusion criteria has been removed (item 4).
In total, four items were added. Did the research questions and inclusion criteria include elements of the PICO (item 1)? Did the author explain why the systematic review was chosen to include the study design type (item 3)? If a meta-analysis was performed, did the authors consider the potential impact of the risk of bias included in the study on the meta-analysis or other integration of evidence (item 12)? Did the author satisfactorily explain or discuss the heterogeneity in the results of the systematic review (item 14)? Finally, AMSTAR-2 removed the “not applicable” and “cannot answer” options in the original AMSTAR instrument, and answered each item with “yes”, “no” and “partial yes”.
Mechanism of SFI on cardiac function and apoptosis
SFI is an intravenous injection made from the extracts of red ginseng and aconite. Modern pharmacological studies shown that the active ingredient of red ginseng in SFI is ginsenoside, which has positive inotropic effect and can enhance myocardial contractility. It can also enhance the body’s ability to resist hypoxia and ischemia, improve the energy metabolism of cardiomyocytes. It also inhibits platelet aggregation to a certain extent. The active ingredient in aconite is normethylaconitine, which is similar to isoproterenol. It can increase the level of cyclic adenosine in cardiomyocytes and enhance atrioventricular conduction and myocardial contractility. At the same time, α-adrenergic receptors can also be excited, which can significantly promote the reduction of coronary cerebral and peripheral vascular resistance by increasing coronary artery and brain blood flow, and improve the situation of myocardial blood and oxygen supply. SFI also had a significant protective effect on myocardial ischemia-reperfusion injury (35). In addition, red ginseng and aconite can also remove oxygen free radicals in blood, inhibit lipid peroxidation, reverse ventricular remodeling and improve heart function (36-38). Caspase 3-mediated apoptosis is related to myocardial injury. Studies have found that SFI could reduce myocardial injury and improve myocardial ultrastructure by inhibiting the expression of Bcl-2, Bax and Caspase 3 proteins, regulate myocardial cell apoptosis, and have a cardiac protective effect (39).
Recommendations for the future based on research outcomes
Based on the results of this study, we found that most SRs were methodological problems. For example, no research plan or registration agreement was provided in any of the literature; it is necessary to make a detailed research plan before the SR, and following this plan may reduce the risk of bias in the process of SR. Therefore, researchers must register agreement on the relevant registration platform [such as PROSPERO (https://www.crd.york.ac.uk/prospero/)] before conducting their SR. Besides, the search strategy was not comprehensive, in addition to the resources obtained in electronic retrieval, gray literature is very important. Researchers should also perform supplementary retrieval, such as professional registries, consultation with experts in related fields, and manual retrieval of other gray literature to fully obtain the research references. At the same time, researchers should make a detailed list of excluded literatures and the reasons for exclusion. Detailed search strategies and exclusion lists are conducive to the repeatability of other studies. Furthermore, the systematic reviewers should use bias risk tools appropriately to comprehensively evaluate the methodological quality of the original literature and evaluate the possible bias caused by confounders, selective bias, exposure and outcome measurement bias, and selective reporting bias; they should then conduct subgroup analysis or regression analysis if necessary. Finally, researchers should clearly explain the source of project funds, the identity of the sponsor, and any conflicts of interest.
Conclusions
The results showed that compared with WM alone, the SFI and WM combined treatment of HF could improve clinical efficacy, quality of life, and cardiac function. However, the methodological and evidence quality of most SRs was poor, so we could not draw a clear conclusion. Judging from the existing results, it is still necessary to explore the efficacy and safety of SFI in the treatment of HF. A high quality SR should be formulated in strict accordance with the items required by the AMSTAR-2 tool, in order to improve the methodological quality, and be explained and elaborated in accordance with the PRISMA list one by one to improve the standardization and transparency of the SR.
Acknowledgments
The authors extend their thanks to Miss Wang for the revision of this article.
Funding: This research was funded by the Key Discipline Construction Project of Sichuan Administration of Traditional Chinese Medicine (202072).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-1073). All authors report funding from the Key Discipline Construction Project of Sichuan Administration of Traditional Chinese Medicine (202072). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016;13:368-78. [Crossref] [PubMed]
- Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail 2014;1:4-25. [Crossref] [PubMed]
- Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30-41. [Crossref] [PubMed]
- Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606-19. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137-61. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Hu SS, Gao RL, Liu LS, et al. The Chinese Cardiovascular Report 2018 is a general review. Chinese Journal of Circulation 2019;34:209-20.
- Zhang J, Zhang YH. Clinical significance of heart failure in patients with chronic heart failure. Chinese Journal of Circulation 2015;30:413-6.
- Bo D, Yue GX, Wang R, et al. Clinical characteristics of 5 traditional Chinese medicine injections in the treatment of heart failure based on meta-analysis literature. China Journal of Chinese Materia Medica 2018;43:4152-62. [PubMed]
- Luo X. Study on pharmacological action of Shenfu injection. Journal of Clinical and Experimental Medicine 2007;6:157, 159.
- Wang ZF, Yu JY, Xie YM. Clinical safety monitoring of Shenfu injection in 30106 patients. China Journal of Chinese Materia Medica 2017;42:2871-6. [PubMed]
- Yang FW, Zou JH, Wang Y, et al. Network Meta-analysis of Chinese medical injections for heart failure. Zhongguo Zhong Yao Za Zhi 2018;43:1247-53. [PubMed]
- Wang KH, Wu JR, Zhang D, et al. Comparative efficacy of Chinese herbal injections for treating chronic heart failure: a network meta-analysis. BMC Complement Altern Med 2018;18:41. [Crossref] [PubMed]
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [Crossref] [PubMed]
- Zhang FY, Shen AM, Zeng XT, et al. Evaluation of quality evaluation tool AMSTAR 2 in systematic evaluation methodology. Chinese Journal of Evidence-Based Cardiovascular Medicine 2018;10:14-8.
- Tao H, Yang LT, An P, et al. Evaluation of quality assessment tool AMSTAR 2 for systematic evaluation of randomized or non-randomized prevention and control research. Chinese Journal of Evidence-Based Medicine 2018;18:101-8.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 2004;4:38. [Crossref] [PubMed]
- Song WT, Cheng FF, Xu L, et al. Chinese medicine shenfu injection for heart failure: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2012;2012:713149 [PubMed]
- Luo HM, He MF, Li KY, et al. Meta-analysis of Shen Fu injection in the treatment of heart failure. Chinese Journal of Traditional Chinese Medicine 1730;2015:1717-9.
- Bin XF, Guo ZH. Treatment of congestive heart failure with Shenfu injection: a systematic review and meta-analysis. Journal of Guiyang College of Traditional Chinese Medicine 2010;32:76-9.
- Wu HT. Systemic review of randomized controlled trials of Shenfu injection in treatment of acute heart failure. Global Journal of Traditional Chinese Medicine 2018;11:1169-76.
- Guo LJ, Wang AZ, Gao F, et al. Efficacy of Shenfu injection for acute heart failure: a meta-analysis. World Chinese Medicine 2020;15:2387-96.
- Jia MX, Yang L, Liu H, et al. Systemic evaluation of the efficacy of Shenfu injection in the treatment of acute left heart failure. Chinese Medicine Emergency 2018;27:797-801.
- Xu PR, Xiao S, Li T, et al. Shenfu injection for heart failure in old patients: a meta-analysis. West China Medical Journal 2013;28:1822-6.
- Ma J, Ye W, Guo JH, et al. Meta-analysis of the treatment of chronic heart failure with ginsenfu injection. Journal of Hubei University of Science and Technology 2017;31:283-8. (Medical Edition).
- Wen JX, He X, Yang YX, et al. Treatment of heart failure with Shenfu injection. Evaluation and Analysis of Drug Use in Chinese Hospital 2017;17:145-51.
- Hou YZ, Mao JY, Wang XL, et al. Shenfu injection for patients with heart failure: a systematic review. Chinese Journal of Evidence-Based Medicine 2011;11:292-9.
- Du H, Dai XH. Meta-analysis of Shenfu injection in the treatment of heart failure. Chinese Journal of Traditional Chinese Medicine 2014;29:3643-6.
- Huang F, Xu HB. Systematic evaluation of Shenfu injection in the treatment of heart failure. Chinese Journal of Hospital Pharmacy 2011;31:1103-8.
- Wang X, Zhao Z, Mao J, et al. Randomized, double-blinded, multicenter, placebo-controlled trial of Shenfu injection for treatment of patients with chronic heart failure during the acute phase of symptom aggravation (Yang and Qi deficiency syndrome). Evid Based Complement Alternat Med 2019;2019:9297163 [Crossref] [PubMed]
- Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol 2009;54:1695-702. [Crossref] [PubMed]
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005;112:e154-235. [PubMed]
- Wang XL, Mao JY, Hou YZ. TCM clinical evaluating method of disease-syndrome, system segment and multidimensional index. Chinese Journal of Integrative Traditional and Western Medicine 2013;2:270-3.
- Wang YY, Li YY, Li L, et al. Protective effects of Shenfu injection against myocardial ischemia-reperfusion injury via activation of eNOS in rats. Biol Pharm Bull 2018;41:1406-13. [Crossref] [PubMed]
- Zi Y, Li ZY. Research overview of Shenfu injection in treating heart failure. Journal of Liaoning University of Traditional Chinese Medicine 2006;4:57-60.
- Liao YH. A new perspective on the treatment of heart failure. Journal of Clinical Cardiovascular Diseases 2005;21:1-2.
- Ran YL. Clinical effect of Shenfu injection on acute exacerbation of chronic heart failure (heart-kidney Yang deficiency type). Hefei: Anhui University of Chinese Medicine, 2017.
- Gu W, Li C, Yin W, et al. Shen-fu injection reduces postresuscitation myocardial dysfunction in a porcine model of cardiac arrest by modulating apoptosis. Shock 2012;38:301-6. [Crossref] [PubMed]
(English Language Editor: J. Jones)



