
Acute appendicitis caused by metastatic gastric adenocarcinoma: a case report and literature review
Introduction
Acute appendicitis (AA) is the most common disease that leads to hospital admission for emergency appendectomy. Less than 3% of all appendectomies are necessitated by appendiceal tumors. Secondary tumors of the appendix are rare and usually originate from ovarian, breast, gastrointestinal, and respiratory tract cancers (1).
Gastric cancer (GC) ranks fifth in terms of incidence and third in mortality worldwide (1). There are notable differences in the Western and Eastern GC populations including etiology, epidemiology, primary tumor site, histopathology, treatment strategies, molecular biological characteristics, and prognoses (2). In the clinic, GC with appendiceal metastasis presenting as AA is rare. The underling mechanism is not clear, and the prognoses of these patients have been discrepant.
Here, we have reported a case of AA due to delayed diagnosis of metastatic gastric adenocarcinoma from China, with evidence of multi-site metastases. The final pathology of endoscopic biopsy and positron emission tomography-computed tomography (PET-CT) confirmed late-stage GC with multi-site metastases which had already advanced beyond the surgical window of opportunity. The patient died about 7 months after appendicectomy. This report includes a summary of 7 previously reported cases in addition to the presented case. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-1098).
Case presentation
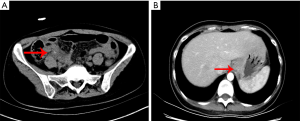
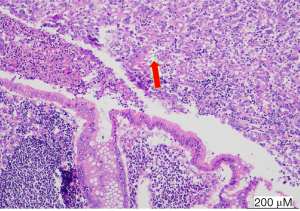
A 33-year-old female was referred to our hospital due to complaining of metastatic right lower quadrant pain, typical appendicitis without any history of tumor. Physical examination showed right lower abdomen tenderness and rebound pain. Preoperatively, the patient underwent computed tomography (CT) (Figure 1A) of the lower abdomen and pelvis, which showed appendix area inflammation effusion indicating AA, the postoperative CT was shown in Figure 1B. She immediately underwent laparoscopic appendectomy for AA. The appendix measured 4.5 cm, was biopsied, and found to contain poorly differentiated adenocarcinoma. Suspecting metastatic cancer (maybe gastrointestinal origin), immunohistochemistry (IHC) revealed CEA(+), CK7 (focal +), CK(+), CK20 (focal +), CD34 (vascular +, tumor bolt), Vimentin(−), CDX2(−), Villin(+), p40(−), ki67 (about70%), Chromogranin A(−), Synaptophysin(−), CD56(−), NSE(−), CD117(−), CA125(−) (Figure 2). Postoperatively, an enhanced CT of the abdomen, pelvis, and an electronic gastroscope was taken, which revealed tissue thickening of the lower gastric esophagus and fundus of stomach (Figure 1B), of which a biopsy was implemented. The corresponding pathology reported a poorly differentiated adenocarcinoma of the gastric fundus with CerbB-2(0) and HP(−). The PET-CT revealed thickening of the stomach wall around the cardia and at the bottom of the stomach; besides, the lesser curvature of the stomach was found located with significant focal FDG (18F fluorodeoxyglucose)-avid lesions. Multiple site metastases were suspected including neck nodes, bilateral hilus of the lung, mediastinum, and retroperitoneum and multiple lymphadenopathies, bilateral ovaries and fallopian, lesser curvature momentum nodules, multiple bone, and midline nodules in the lower abdomen and abdominal wall. The corresponding level of CEA was 7.93 ng/mL (Figure 3), CA125 was 186.2 U/mL, and CA19-9 was 67.58 u/mL at the first check. The patient took the chemotherapeutic drug S-1 (50 mg bid d1–14, Q3w) orally from 30 January 2020 to 27 March 2020 according to the oncologist’s suggestion. The patient then received 5 cycles of chemotherapy (oxaliplatin 150 mg d1+ S-1 50 mg d1–14, Q3w) post-operatively after 25 cycles of pelvic radiation therapy. Considering the disease progression (ascites and intestinal obstruction), second-line chemotherapy (albumin-bound paclitaxel 0.2 g + raltitrexed 4 mg) were adopted alternatively. The patient died of circular respiratory failure on 16 September 2020. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and any accompanying images.
Discussion
The AA is the most common general surgical emergency for appendectomies. Appendiceal tumors, occurring in less than 3% of all appendectomies, are rarely associated with clinical manifestations; they are frequently recognized either during an operation or via pathology. Malignant tumors of the appendix include carcinoids (most common type), followed by goblet-cell carcinoid and lymphoma. In the literature review which consisted of 80,698 biopsies of appendectomy, 7 cases were confirmed as secondary adenocarcinoma, accounting for 0.001239% (3), and 11 cases of primary adenocarcinoma were diagnosed (3).
A survey showed that the most common primary origin of the secondary tumor was the ovary, followed by colorectal and digestive tracts. Another reported that primary breast carcinoma was the most common primary origin followed by urogenital, gastrointestinal, and respiratory cancers (3).
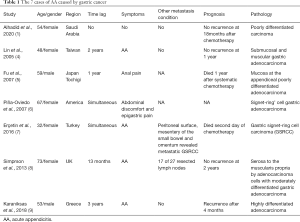
It is rare that AA is caused by metastasis of GC to appendix, and it is usually detected intra-operatively in such patients. Generally, pathology diagnosed the metastasis, while subsequent work up revealed the origin of the primary tumor. The diagnosis of the cases mostly depended on postoperative pathology (1,4-9) (Table 1). Notably, the report of Almana et al. (1) indicated GC history before AA, and Lin et al. reported that recurrent gastric adenocarcinoma was the cause of AA (4). Fu et al. (5) reported that colonoscopy had revealed metastasis from GC 1 year previously. The case in Piña-Oviedo et al.’s report (6) showed appendix metastasis after laparotomy for gastric signet-ring cell adenocarcinoma (GSRCC) that was confirmed during endoscopy bioscopy. Simpson (8) reported a case admitted as AA 13 months following surgical resection of gastric malignancy. Meanwhile, the case report by Michael Karanikas (9) showed that the patient had a history of GC 3 years before AA. Only the patient examined by Erçetin et al. (7) did not have a history of GC, and it was detected intra-operatively as suspicious greyish-white nodules. Our case had no history of GC and was diagnosed with appendix metastasis postoperatively after subsequent work up.
Full table
Currently, the 5-year survival rate of GC patients is less than 20%, due to the recurrence and metastasis of GC. The principles for the surgery of GC are endoscopic mucosal resection (EMR) for Tis/T1a and gastric resection with D2 lymph node dissections for T1b-T3 (2) [National Comprehensive Cancer Network (NCCN) guidelines]; appendectomy in every gastrectomy is not currently recommended. Not all metastatic tumors caused appendicitis, thus the real incidence of appendiceal metastasis in GC remains unknown. The benefit-risk ratio of appendectomy in every gastrectomy should be further explored and studied. In some malignancies, resection of the organ that is secondarily involved is known to improve survival rates (such as in metastatic colorectal liver cancer, pancreatic metastasis of renal cell tumor, and so on). Conversely, appendectomy does not seem to improve survival rate in patients with secondary tumors of the appendix jenny (7). Guidelines on the management of GC cases are also needed in relation to routine appendectomy.
Most GC patients have had adjacent organ or distant metastases, which are the main cause of death in GC patients. The intrinsic determinants of GC-specific metastasis are still unclear. Finding specific metastasis-related genes important for GC metastasis to the liver, lung, and appendix is a priority for future research (10). Candidate biomarkers are needed for future research as indicators of GC metastasis to the appendix and other organs to guide surgical decisions.
After review, the route (lymphatic metastasis, serous membrane metastasis, or hematogenous metastasis) of metastasis to the appendix was still ambiguous. In the report by Lin et al., the patient showed appendix involvement only (4), and Erçetin et al. reported a metastatic GSRCC on the serous surface of the appendix that was detected in samples obtained from the appendix, implying a serous membrane-metastatic route (7). Additionally, Almana et al. reported an appendix sample showing metastatic poorly differentiated carcinoma with GC (confirmed as invasive adenocarcinoma, diffuse type with signet ring morphology) with mural involvement (3). Karanikas [2018] reported a gastric adenocarcinoma with 17 of 27 resected lymph nodes positive for metastasis 3 years before the AA (9), suggesting that tumor cells can travel via the lymphatic system (8). A literature review showed that metastatic carcinoma of the appendix could result from primary breast carcinoma (7), suggesting that the tumor cells may metastasize to the distant appendix through either blood or lymph. This case showed multiple metastases including lymphadenopathy, bilateral ovaries and fallopian tubes, and lesser curvature of the momentum, which hinted that the metastasis to the appendix may have resulted from the profound tumor invasion ability and the potential route was membrane-metastatic and lymphatic.
The median survival after diagnosis of the secondary tumor of the appendix was 22.6 months (7). The prognosis of the cases remained discrepant (Table 1) for the pathological type, clinical stage, and corresponding treatment plan of the primary tumor. The cases reported by Simpson, 2013 (8) and Lin, 2005 (4) had promising prognoses, with no recurrence detected at 1 year follow-up, and Almana et al. (3) detected no recurrence in their case at 18 months after chemotherapy. Meanwhile, the patient reported on by Karanikas (9) experienced recurrence after 4 months and in Piña-Oviedo S’s (6) report the patient died 1 year after systematic chemotherapy. The patient reported by Erçetin et al. (7) died on the second day of chemotherapy. Follow up was not reported on by Michael Karanikas (9). At our center, we follow up patients for 7 months, and the current case died 7 months after receiving appendectomy surgery and systematic chemotherapy. The prognosis should consider the pathology and general condition of the patient. Regarding pathology, Karanikas et al. (9) and Simpson et al. (8) reported that AA pathology was involved with moderately differentiated gastric adenocarcinoma, and the other 5 cases revealed poorly differentiated adenocarcinoma including 1 report from Piña-Oviedo et al. (6) in which the patient was diagnosed with GSRCC. Here, we reported a patient with AA caused by metastasis from poorly differentiated adenocarcinoma of the gastric fundus (HER2 negative), who was shown to be multiple metastasis. The prognosis of AA due to metastasis of GC should refer to the GC prognosis, tumor, node, metastasis (TNM) stage, surgical procedures, pathology, and the IHC. Immunosuppressive drugs are currently approved for patients who have failed at least two prior lines of systemic chemotherapy in metastatic GC (11). However, the patients did not survive for the immune therapy.
Conclusions
Metastatic GC may rarely manifest itself with AA. It is often diagnosed after appendectomies in patients with AA; 6 of 8 cases reviewed in this report had a history of GC. The survival of the metastatic GC was discrete. The review of these scattered cases triggered doubt about the true incidence of appendix metastasis in gastric tumors, especially asymptomatic metastases, and the benefit-risk ratio of appendectomy for every patient with GC. The prognoses of these patients require systematic evaluation, and there is a need for guidelines on the management of such cases.
Acknowledgments
This work was supported by the colleagues in our center.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-1098
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-1098). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alhadid D, AlShammari A, Almana H, et al. Missed gastric cancer metastasis to the appendix: case report and literature review. Am J Case Rep 2020;21:e920010 [Crossref] [PubMed]
- Wang FH, Shen L, Li J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019;39:10. [Crossref] [PubMed]
- Akbulut S, Tas M, Sogutcu N, et al. Unusual histopathological findings in appendectomy specimens: a retrospective analysis and literature review. World J Gastroenterol 2011;17:1961-70. [Crossref] [PubMed]
- Lin CY, Huang JS, Jwo SC, et al. Recurrent gastric adenocarcinoma presenting as acute appendicitis: a case report. Int J Clin Pract Suppl 2005;89-91. [Crossref] [PubMed]
- Fu K, Horimatsu T, Sano Y, et al. Metastasis to the appendix from gastric cancer detected incidentally on colonoscopy. Endoscopy 2007;39:E17 [Crossref] [PubMed]
- Piña-Oviedo S, Del Valle L, de Leon-Bojorge B, et al. 'Signet-ring' cell gastric adenocarcinoma metastatic to a neurogenous hyperplasia of the appendix. Histopathology 2007;50:663-5. [Crossref] [PubMed]
- Erçetin C, Dural AC, Özdenkaya Y, et al. Metastatic gastric signet-ring cell carcinoma: A rare cause of acute appendicitis. Ulus Cerrahi Derg 2015;32:140-4. [Crossref] [PubMed]
- Simpson GS, Mahapatra SR, Evans J. Incidental complete excision of appendiceal gastric cancer metastasis. J Surg Case Rep 2013;2013:rjt080 [Crossref] [PubMed]
- Karanikas M, Kofina K, Markou M, et al. Acute appendicitis as the first presentation of appendiceal metastasis of gastric cancer-report of a rare case. J Surg Case Rep 2018;2018:rjy208 [Crossref] [PubMed]
- Dong YD, Yuan YL, Yu HB, et al. SHCBP1 is a novel target and exhibits tumor-promoting effects in gastric cancer. Oncol Rep 2019;41:1649-57. [PubMed]
- Sundar R, Huang KK, Qamra A, et al. Epigenomic promoter alterations predict for benefit from immune checkpoint inhibition in metastatic gastric cancer. Ann Oncol 2019;30:424-30. [Crossref] [PubMed]
(English Language Editor: J. Jones)



